Summary
The utility of cellular and tissue-based wound care products in soft-tissue facial reconstruction is dependent on careful patient selection and wound management.
Cellular and tissue-based products can be used to temporize all wounds and can provide final reconstruction for selected wounds.
Extracellular matrices have the potential to progress to wound healing without additional procedures.
Acellular dermal matrices require an additional procedure to achieve final wound healing.
4.1 Algorithm
4.1.1 General Considerations
Cellular and tissue-based wound products have been available for decades (▶ Fig. 4.1). Currently, hundreds of products are available all with different degrees of efficacy but more importantly all with different degrees of promised efficacy that often does not match reality. There are tremendous marketing forces that drive these often expensive products that the user has no recourse for failure and again the patient is the one who suffers.
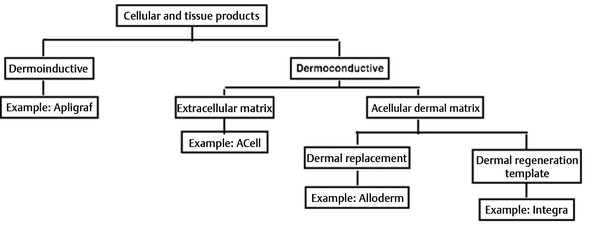
Fig. 4.1 Algorithm for choice of CTP (cellular and tissue-based product).
However, in their current state, many, many products are very useful and can help restore patients to normalcy without donor site morbidity or extensive operative procedures. It is up to the practitioner to develop critical analytic skills on what products work and become proficient in product selection and use.
In very general terms, cellular and tissue-based products (CTPs) fall into two general categories: dermoinductive and dermoconductive. 1 Dermoinductive CTPs include a wide range of products that include Apligraf, TheraSkin, Dermagraft, and Epicel. 1 They are products that actually provide living cells into a wound and are designed to stimulate the activity of either new tissue growth or tissue granulation within a wound. 2 These products are uniformly expensive and require distinctive handling skills for their successful use. For all practical purposes, given the robust vascularity of head and neck patients, dermoinductive wound healing products provide little utility and will not be described further. A much broader category are dermoconductive products. These include Integra, GRAFTJACKET, Oasis, AlloDerm, and ACell. These products provide scaffolding within a healing wound that allows cells from surrounding tissue to migrate across the wound and create a neodermis. 2, 3 The advantage of this scaffolding is that it both promotes tissue and growth and is ultimately designed to provide tissue ingrowth without formation of a scar. 3 Dermoconductive products can be further subdivided into three general categories and although they are not scientifically rigorous definitions, the divisions can help guide product selection. The general categories include acellular dermal matrix, which can result in progression to complete wound healing; extracellular dermal matrix, which can also result in complete wound healing; and dermal regeneration template, which requires an additional skin grafting procedure to achieve full wound closure, the only one that will be described here—Integra. 4, 5, 6
Integra has been in active use since the early 1980s when it was originally designed to facilitate wound healing in large open wounds particularly from burn excisions. It is a regenerating two-dimensional structure or a neodermis in which it is composed of a layer of bovine collagen crosslink with glycose glycans covered by a silastic membrane. 5, 6 So it is a bilaminar structure. The collagen is specifically shark cartilage and the vertical design provides for infiltration of host cells into the collagen matrix or scaffolding and then this forms a “neodermis” over the course of 3 to 6 weeks. The silicone outer layer lifts off and at this point a thin split-thickness skin graft is required for completion of wound healing. Integra has been described by multiple authors for use in head and neck reconstruction and has several unique roles, particularly in scalp reconstruction where it can provide coverage over exposed bone, whereas the acellular dermal matrix or extracellular dermal matrix cannot reliably provide the same. 4, 5, 6 The use of Integra does involve a fairly steep practitioner-based learning curve and is expensive.
4.2 Integra
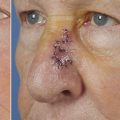
The first category of dermoconductive products are dermal regeneration templates, of which only Integra will be discussed. Integra is currently available as a mesh bilayer wound matrix in a large variety of sizes. It is placed over a wound bed that is often not suitable for grafting with exposed bone and cartilage. It is secured in place either as a simple bolster or with the use of negative pressure wound treatment (NPWT) sponge. After 5 to 7 days, the initial wound dressing, either matrix or NPWT wound sponge is removed and then the patient is allowed to shower and essentially resume normal activity and by design, the Integra has no requirements for further wound care at this point. Over the course of 3 to 8 weeks, depending on the wound bed, as well as ultimate patient factors, the Integra heals and the silicone sheeting is lifting off by the ingrowing underlying soft tissue, and the resulting wound bed is now suitable for grafting. Originally described for use with split-thickness grafting only (0.8-mm thickness), Integra has now been described by multiple authors as being useful in thicker split-thickness grafting and even full-thickness grafting. 6 One distinct characteristic with Integra should be mentioned. As the soft-tissue ingrowth occurs around week 2 or 3, a creamy exudate will develop underneath the silicone sheeting and it can be easily confused with an infected process. It is incumbent upon the practitioner to understand that this is a normal occurrence and without additional signs of soft-tissue infection is likely not an infective process and can be simply monitored and then the exudate removed prior to grafting. The advantages of Integra at this point are multifold. It can obviate the need for involved multistage local or free-flap procedures. 5 It can serve as a viable placeholder in a patient undergoing staged surgical resection until the pathological results return. Authors have described Integra to be initially placed prior to reconstruction while waiting for pathological clearance for oncologic margins of resected specimen. At this point, final reconstruction can be pursued or further cancer resection can be completed if necessary (▶ Fig. 4.2, ▶ Fig. 4.3, ▶ Fig. 4.4, ▶ Fig. 4.5, ▶ Fig. 4.6, ▶ Fig. 4.7, ▶ Fig. 4.8).
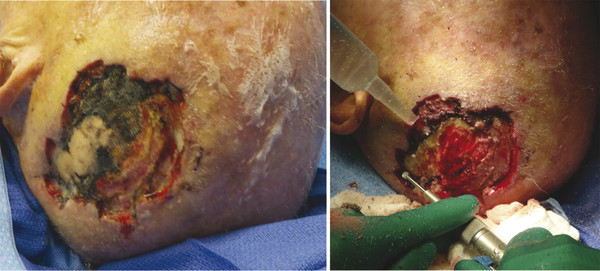
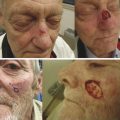
Fig. 4.2 An 89-year-old male with multifocal squamous cell carcinoma of the scalp status postoperative resection. He presented with chronically exposed calvarium. Potentially morbid wound treated with simple bone burring and Integra placement done as an outpatient procedure under IV (intravenous) sedation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree