40 Case Presentations with Expert Discussions
CASE 1
Pregnant and Requesting Elective Cesarean Section: Point-counterpoint
Discussant: Matthew D. Barber
Patient-choice or elective cesarean section has been a topic of much discussion and heated debate of late in the lay press and in the medical literature. It is of particular interest to urogynecologists and pelvic surgeons because much of the focus has been on the potential to prevent pelvic floor disorders by avoiding vaginal delivery. When comparing modes of delivery, obstetricians, historically, have compared the short-term morbidity and mortality of vaginal delivery to those of cesarean section. Such a comparison strongly favors vaginal delivery and would seem to preclude elective cesarean section as a viable option. However, for the scenario listed previously, this traditional risk comparison is flawed. First, with the widespread adoption of regional anesthesia, prophylactic antibiotics, and antithrombotic compression devices, the risk of cesarean section has declined. Second, it is not the risk of cesarean section in general that should be considered, but rather the risks of elective cesarean section without labor. A cesarean section that occurs before the onset of labor and before or early after rupture of membranes has a substantially lower risk of morbidity and mortality than cesarean section performed after laboring for several hours or cesarean section performed emergently. Current evidence suggests that a scheduled cesarean section performed before the onset of labor has a mortality rate similar to that of a vaginal delivery (Lewis et al., 2001). Third, not all women who attempt a vaginal delivery are able to do so successfully. Approximately 10% to 15% will require an operative vaginal delivery, and 10% to 25% will require a cesarean section after laboring for some period of time, each of which have an increased risk of morbidity. Thus, it is not the risk of vaginal delivery that should be considered, but rather the risk of a planned vaginal delivery or trial of labor. Fourth, traditional risk comparisons have focused on short-term risks of each mode of delivery and have failed to account for long-term morbidity or quality-of-life considerations. Therefore, when counseling this patient, the risks and benefits of a scheduled cesarean section performed before labor should be weighed against those of a trial of labor, and both short and long-term morbidity should be considered.
Unfortunately, there are no randomized trials directly comparing purely elective cesarean section to trial of labor to provide an unbiased comparison of risks. Perhaps the best available comparison is the Term Breech Trial, which randomized women at term with breech presentation to “planned vaginal delivery” versus “planned cesarean section.” The overall morbidity rates in this trial, including infection, postpartum hemorrhage, and blood transfusions, were not significantly different between the two modes of delivery (OR 1.24 [95% CI 0.78 to 1.95]) (Hannah et al., 2000). Regarding fetal risks, the available evidence suggests that cesarean section performed before labor may decrease the risk of cerebral hemorrhage and brachial plexus injury but increase the risk of transient tachypnea of the newborn (TTN) when compared to vaginal delivery (Morrison et al., 1995; Towner et al., 1999). However, the risk of TTN is minimized, if the cesarean section is performed at 39 weeks of gestation or greater (Morrison et al.).
The long-term morbidity of vaginal delivery is primarily the risk of future pelvic floor disorders. Although the etiology of most pelvic floor disorders is undoubtedly multifactorial, vaginal childbirth is an established risk factor for each of the most prevalent disorders, including stress urinary incontinence (SUI), anal incontinence, and pelvic organ prolapse, particularly in reproductive aged women. Data from the study by Rortveit et al. (2003) suggest that the attributable risk of vaginal delivery for the development of moderate to severe SUI is approximately 50%. Lukacz et al. (2005) recently reported that the attributable risk of vaginal delivery for stress incontinence, overactive bladder (OAB), pelvic organ prolapse, and anal incontinence ranges from 37% to 46%. The authors estimated that five unlabored cesarean sections would be needed to prevent one pelvic floor disorder. Thus, elective cesarean section moderately reduces, but does not eliminate, the risk of a woman developing one or more pelvic floor disorder(s) during her life. The long-term morbidity of elective cesarean section is primarily related to the risks associated with repeat cesarean sections and includes an increased risk of placenta previa, placenta accreta, and uterine dehiscence/rupture. The risk of each of these conditions increases with each subsequent cesarean section, so a discussion of planned family size should be an important consideration when counseling a woman about mode of delivery.
Hannah ME, Hannah WA, Hawson SA, et al. Planned caesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Term Breech Trial Collaborative Group. Lancet. 2000;356:1375.
Lewis G, Drife J, Botting B, et al. Why Mothers Die, 1997–1999: The Confidential Enquiries into Maternal Deaths in Great Britain. London: RCOG Press, 2001.
Lukacz ES, Lawrence JM, Nager CW, et al. The effect of pregnancy and mode of delivery on pelvic floor dysfunction: an epidemiologic study. J Pelvic Surg. 2005;11(Suppl 1):S2.
Morrison JJ, Rennie JM, Milton PJ. Neonatal respiratory morbidity and mode of delivery at term: influence of timing of elective caesarean section. Br J Obstet Gynaecol. 1995;102:101.
Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S. Vaginal delivery parameters and urinary incontinence: the Norwegian EPINCONT study. Am J Obstet Gynecol. 2003;189:1268.
Towner D, Castro MA, Eby-Wilkins E, et al. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709.
CASE 2
Procidentia and Uterine Preservation
Case: The patient is a 35-year-old para 4 woman with severe pelvic pressure and mild stress incontinence. She has had a tubal ligation. On examination, there is complete uterine procidentia with eversion of the anterior vaginal wall (Fig. 40-1). Subtracted urodynamics revealed mild urodynamic stress incontinence (USI), without evidence of intrinsic sphincter deficiency (ISD). She is not interested in using a pessary and insists on uterine preservation.
Discussant: G. Rodney Meeks
I would perform reconstructive surgery and accept the stipulation of uterine preservation. If the patient had not completed childbearing, I would have some reservations. I, personally, have not seen corrective surgery survive a subsequent pregnancy. However, there is literature documenting that delivery can occur with the repair remaining intact (Kovac and Cruikshank, 1993). The patient’s complaints of pelvic pressure, urinary incontinence, and uterovaginal prolapse must be addressed. Before surgery, I would assess all potential sites of pelvic organ prolapse with the uterine prolapse reduced. I would determine whether the anterior vaginal wall had a central defect or a lateral detachment. Certainly, some lateral detachment is likely with complete prolapse, and an enterocele is often present. I would assess the posterior wall for detachment from the perineal body and from the vaginal apex, as well as the rectovaginal fascia for site-specific defects.
The third approach involves anchoring the uterus and cervix, or both, to the sacrum with either natural or synthetic materials. A video publication details this technique (Cholhan, 1998). Recent reports have compared results with and without hysterectomy (Barranger et al., 2003; Leron and Stanton, 2001). Outcomes are comparable in both groups. Another abdominal option, which may lessen the risk of complications, is to suspend the uterine fundus to the pectineal ligament with synthetic mesh (Joshi, 1993).
The fourth approach is to anchor the cervix to the sacrospinous ligaments. Richardson et al. (1989) described a transvaginal approach in which the uterosacral ligaments were attached to the sacrospinous ligaments. A needle procedure was used to treat incontinence. The authors described a high degree of success with this technique and also reported that subsequent pregnancies occurred without recurrence of the prolapse. Two recent articles have compared hysterectomy and hysteropexy when performed in combination with sacrospinous ligament suspension for uterovaginal prolapse (Hefni et al., 2003; Maher et al., 2001). Results are comparable with both techniques.
I remain a proponent of the vaginal approach to prolapse repair and would recommend it for this patient. I would use the technique described by Richardson et al. (1989) to suspend the uterus, but I would substitute a transvaginal tape for USI. I would also repair the enterocele, reattach the pubocervical fascia to its lateral attachments, and perform a site-specific posterior colporrhaphy.
Barranger E, Fritel X, Pigne A. Abdominal sacrohysteropexy in young women with uterovaginal prolapse: long-term follow-up. Am J Obstet Gynecol. 2003;189:1245.
Cholhan HJ. Transabdominal uterovaginal suspension: an alternative to hysterectomy for uterovaginal prolapse. Washington, DC: ACOG Audiovisual Library, 1998.
Hefni M, El-Toukhy T, Bhaumik J, Katsimanis E. Sacrospinous cervicocolpopexy with uterine conservation for uterovaginal prolapse in elderly women: an evolving concept. Am J Obstet Gynecol. 2003;188:645.
Joshi VM. A new technique of uterine suspension to pectineal ligaments in the management of uterovaginal prolapse. Obstet Gynecol. 1993;81(Pt 1):790.
Kovac SR, Cruikshank SH. Successful pregnancies and vaginal deliveries after sacrospinous uterosacral fixation in five of nineteen patients. Am J Obstet Gynecol. 1993;168:1778.
Leron E, Stanton SL. Sacrohysteropexy with synthetic mesh for the management of uterovaginal prolapse. BJOG. 2001;108:629.
Maher CF, Cary MP, Slack MC, et al. Uterine preservation or hysterectomy at sacrospinous colpopexy for uterovaginal prolapse? Int Urogynecol J. 2001;12:381.
Richardson DA, Scotti RJ, Ostergard DR. Surgical management of uterine prolapse in young women. J Reprod Med. 1989;34:388.
Discussant: Christopher F. Maher
The Manchester repair has largely been abandoned due to recurrence of prolapse in excess of 20% in the first few months, decrease in fertility, and pregnancy wastage as high as 50%. In addition, future sampling of the cervix and endometrium can be difficult due to vaginal reepithelialization or cervical stenosis. The sacrospinous hysteropexy is a safe and effective procedure as compared to vaginal hysterectomy and sacrospinous colpopexy for uterine prolapse. Two comparative studies of 165 women, with at least a mean 2-year review, are available and demonstrate that operating time, blood loss, and complications are reduced in the hysteropexy (versus hysterectomy) group, with success rates of 90% being reported (Hefni et al., 2003; Maher et al., 2001b). Only limited data are available on pregnancy outcome following sacrospinous hysteropexy, especially since Hefni et al. (2003) who contributed 109 women to the literature, reported in women only over 60 years. Seven pregnancies have been reported with two women (29%) undergoing further prolapse surgery, one each following vaginal and cesarean delivery (Kovac and Cruikshank, 1993; Maher et al., 2001b).
Alternative abdominal approaches include laparoscopic suture hysteropexy and sacral hysteropexy. We described the laparoscopic suture hysteropexy in which the plicated uterosacral ligaments and cardinal ligaments are resutured to the cervix (Maher et al., 2001a). The objective success rate was 79% after an average of 12 months. Two women completed pregnancies; both delivered abdominally and reported no recurrence of prolapse. The success rate of this surgery may be improved by incorporating partial cervical amputation to the surgery because cervical elongation rather than uterine prolapse represented a significant proportion of the failed group. The operation is simple, effective, and uses native tissue. I would use this procedure in those women considering future pregnancy and in those with mild to moderate uterine prolapse undergoing other pelvic floor surgery. This surgery would not be considered for the women with total uterine procidentia in whom the integrity of the uterosacral ligaments is suspect.
Several authors have reported objective success rates of over 90% with sacral hysteropexy (Barranger et al., 2003; Leron and Stanton, 2001), in which mesh secures the cervix to the sacrum. In a randomized controlled trial comparing sacral hysteropexy with vaginal hysterectomy and repair, Roovers et al. (2004) reported a significantly higher reoperation rate for prolapse in the hysteropexy group. Individual site-specific results were not available, but one suspects that the incorporation of a full anterior and posterior mesh extension as used in the sacral colpopexy after hysterectomy would be difficult and may account for the lower success rate. Younger women who subsequently require hysterectomy or develop future upper vaginal prolapse will be problematic with the removal of the mesh from the cervix and sacrum being challenging. Although mesh introduced abdominally to the pelvis is associated with relatively few mesh complications, I remain uncomfortable with the introduction of mesh to the pelvis for primary prolapse when alternative native tissue repairs exist with equivalent success rates.
Barranger E, Fritel X, Pigne A. Abdominal sacrohysteropexy in young women with uterovaginal prolapse: long-term follow-up. Am J Obstet Gynecol. 2003;189:1245.
Hefni M, El Toukhy T, Bhaumik J, Katsimanis E. Sacrospinous cervicocolpopexy with uterine conservation for uterovaginal prolapse in elderly women: an evolving concept. Am J Obstet Gynecol. 2003;188:645.
Kovac SR, Cruikshank SH. Successful pregnancies and vaginal deliveries after sacrospinous uterosacral fixation in five of nineteen patients. Am J Obstet Gynecol. 1993;168:1778.
Leron E, Stanton SL. Sacrohysteropexy with synthetic mesh for the management of uterovaginal prolapse. Br J Obstet Gynaecol. 2001;108:629.
Maher CF, Carey MP, Murray CJ. Laparoscopic suture hysteropexy for uterine prolapse. Obstet Gynecol. 2001;97:1010.
Maher CF, Cary MP, Slack MC, et al. Uterine preservation or hysterectomy at sacrospinous colpopexy for uterovaginal prolapse? Int Urogynecol J. 2001;12:381.
Roovers JP, van der Vaart CH, van der Bom JG, et al. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. Br J Obstet Gynaecol. 2004;111:50.
CASE 3
Cystocele and Potential Stress Incontinence
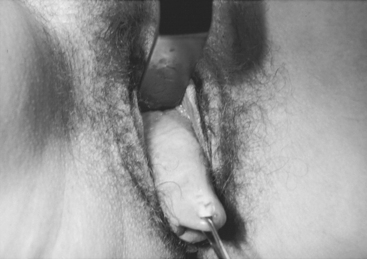
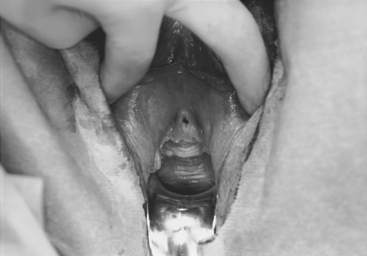
Case: A 49-year-old para 5 woman complains of pelvic pressure and the feeling that she does not completely empty her bladder. She had an anterior colporrhaphy (without hysterectomy) 7 years ago. On examination, she has recurrent anterior vaginal wall prolapse that descends beyond the hymen, with straining in the supine position (Fig. 40-2). The cervix descends to the hymen, and a small enterocele and rectocele are also noted. On spontaneous uroflowmetry, the patient voided 240 mL, with a 10-mL postvoid residual urine volume. Time to void was 42 seconds with a maximum flow rate of 12 mL/sec. Filling subtracted cystometry showed a stable cystometrogram to a maximum capacity of 440 mL. Despite numerous provocative maneuvers in the standing position, no stress incontinence could be demonstrated. A static maximum urethral closure pressure (MUCP) with prolapse unreduced was 53 cm H2O. The prolapse was then gently reduced using a Sims’ speculum (Fig. 40-3), and leakage was demonstrated with coughing and straining, with a Valsalva leak point pressure (LPP) of 45 cm H2O and MUCP of 30 cm H2O. The patient desires surgical correction of her prolapse.
Discussant: Richard C. Bump
Potential Stress Incontinence
It is well recognized that women with advanced stages of pelvic organ prolapse rarely complain of the symptom of stress urinary incontinence (SUI). Advanced anterior segment prolapse, when accentuated by stress, descends and mechanically obstructs the urethra, preventing the occurrence of SUI (Bump et al., 1988). Preoperative reduction of the prolapse, by using devices such as a pessary, a vaginal pack, or a speculum prevents this stress-activated urethral obstruction and reveals so-called potential stress incontinence in 36% to 80% of women with advanced prolapse (Bump et al., 1996). Although barrier testing has been suggested for determining which women should have a urethropexy or sling procedure performed concurrently with prolapse correction surgery, the predictive value of these barrier tests is poor as long as the prolapse surgery addresses the support defect of the urethra and bladder neck that is usually part of the anterior segment prolapse (Bump et al., 1996). In a randomized prospective comparison of needle urethropexy versus bladder neck endopelvic fascia plication, for the prevention of stress incontinence in women who underwent vaginal reconstruction for stage III or IV prolapse, preoperative prolapse reduction testing predicted USI in 67% (10 of 15) who underwent the latter procedure; however, USI was observed in only 7% (1 of 15) at 6 months (Bump et al., 1996). In the entire study population, ISD was predicted in 25% (8 of 32) but was observed postoperatively in only two subjects (6%), of whom one had been predicted preoperatively, although no prophylactic slings were performed. In this study, the positive predictive value of barrier testing was 20% for urodynamic stress incontinence (USI) and 12.5% for ISD. To put the risk of potential incontinence into some perspective, one should remember that the risk of de novo USI after vaginal prolapse surgery (7% to 10%) is lower than the risk of persistent USI after a Burch colposuspension or sling performed to cure USI (8% to 15%).
A recently completed randomized clinical trial confirmed the predictable risk of surgically ignoring the support defect of the urethra and bladder neck that usually accompanies advanced stage pelvic organ prolapse at the time of abdominal sacral colpopexy. In this trial, women with advanced pelvic organ prolapse without symptoms of SUI who underwent abdominal sacrocolpopexy were randomly assigned to concomitant Burch colposuspension or to no Burch colposuspension. Three months after surgery, women in the no Burch group were more likely to report bothersome symptoms of stress incontinence than those in the Burch group who had stress incontinence (24.5% vs 6.1%, P < .001; Brubaker et al., 2006).
Route of Prolapse Correction Surgery
Several considerations have an impact on my choice of surgical route for reconstructive surgery, including the precise defects responsible for prolapse, the cause of the defects, whether the inciting and promoting events are continuing processes, and the patient’s desires and expectations. About 60% of women whom I see with prolapse have discrete endopelvic fascia defects and low-risk profiles for recurrence; in this situation, I perform a vaginal route anatomic repair that corrects all defects (inferior, lateral, superior, and midline) of the indigenous tissues. However, there are circumstances that I think compromise the longevity of these repairs, which are intended to realign the pelvic organs over a relatively normal pelvic floor and to withstand normal physical stresses. Vaginal approaches using endogenous tissues depend on normal pelvic floor muscle support, and I avoid them in women with poor muscle function following attempts at pelvic floor rehabilitation or in women with overwhelming neuromuscular dysfunction, such as spinal cord injuries. Extreme attenuation of native tissues may also compromise the success of these repairs, and I will use mesh or fascial substitutions for the endopelvic fascia in these situations, via either an abdominal or vaginal approach. Finally, women with rapidly recurrent prolapse, extremely active lifestyles, significant perineal descent, or chronic ongoing causes for prolapse (e.g., obesity, refractory constipation, chronic obstructive pulmonary disease) require a repair with more strength than is offered by my anatomic vaginal repair using endogenous tissues. Under these circumstances, I perform a compensatory repair that will include an abdominal sacral colpoperineopexy, combined usually with a Halban culdoplasty, retropubic paravaginal repair and bladder neck suspension, and often with a perineal reconstruction and distal posterior fascial repair and reattachment. However, early reports of transvaginal tension-free meshes to replace the endopelvic fascia anteriorly, posteriorly, and/or superiorly suggest that these evolving techniques may have comparable efficacy with reduced acute morbidity in these patients who are at high risk for recurrent prolapse.
Brubaker L, Cundiff GW, Fine P, et alfor the Pelvic Floor Disorders Network. Abdominal sacrocolpopexy with Burch colposuspension to reduce stress urinary incontinence. N Engl J Med. 2006;354:1557.
Bump RC, Fantl JA, Hurt WG. The mechanism of urinary continence in women with severe uterovaginal prolapse: results of barrier studies. Obstet Gynecol. 1988;72:291.
Bump RC, Hurt WG, Theofrastous JP, et al. Randomized prospective comparison of needle colposuspension versus endopelvic fascia plication for potential stress incontinence prophylaxis in women undergoing vaginal reconstruction for stage III or IV pelvic organ prolapse. Am J Obstet Gynecol. 1996;175:326.
Discussant: Linda Brubaker
A final decision regarding the specific reconstructive procedures may depend on the decision to place a concomitant continence procedure. A recently completed randomized clinical trial of women with stages II to IV prolapse, without symptoms of stress incontinence, suggests that when such women undergo sacrocolpopexy without concomitant continence procedures, approximately 40% develop stress incontinence symptoms (Brubaker et al., 2006). Approximately 1 in 4 had moderate or severe bother from stress incontinence symptoms. These risks are reduced about 20% by the concomitant placement of a Burch colposuspension. Whether these risks are the same at the time of a transvaginal reconstruction without concomitant continence procedures is unknown. Most surgeons avoid the combination of vaginal reconstruction and the Burch colposuspension, instead favoring a synthetic tape sling, such as transvaginal tape (TVT). Although there is a randomized trial demonstrating the comparable efficacy of the Burch colposuspension and TVT, we do not have this information for women with prolapse because they were excluded from that trial (Ward and Hilton, 2002).
Preoperative urodynamic testing, with prolapse reduction, is not as useful as formerly thought. In women without symptoms of stress incontinence, prolapse repair alone may unmask postoperative symptoms of stress incontinence. Most clinicians had a favorite preoperative method of testing women with the intent of predicting the need for a concomitant continence procedure. Information from the CARE trial suggests that reduction with swabs most closely mimics the postoperative situation in women without a concomitant procedure. Although asymptomatic women may demonstrate SUI with prolapse reduction, there is a 20% absolute risk reduction with Burch procedure (in women who undergo sacrocolpopexy), regardless of the findings of such testing.
Brubaker L, Cundiff GW, Fine P, et al. Abdominal sacrocolpopoxy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med. 2006;354:1557.
Ward K, Hilton P. Prospective multicentre randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. United Kingdom and Ireland Tension-free Vaginal Tape Trial Group. Br Med J. 2002;325:67.
CASE 4
Evaluation and Management of Complete Vaginal Eversion
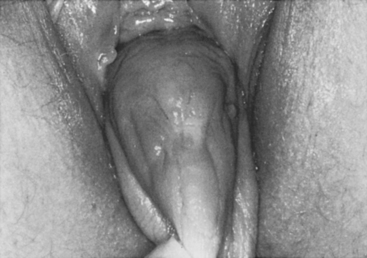
Case: The patient is a 56-year-old woman with severe pelvic pressure. She had an abdominal hysterectomy and retropubic repair 20 years ago. She had a history of recurrent stress incontinence; however, over the last 2 years, this resolved, and she currently has to reduce her prolapse to empty her bladder. Complete vaginal eversion was found on examination (Fig. 40-4). Filling cystometry revealed an uninhibited detrusor contraction at maximum capacity, which was less than 200 mL.
Discussant: Jeffrey L. Cornella
A percentage of patients with vaginal prolapse may sustain urinary retention secondary to obstruction from prolapse. Similarly, some patients who demonstrate SUI early in their history may have resolution of symptoms as the prolapse enlarges and subsequently obstructs the urethra, as appears to have occurred in this patient. If these patients do not receive a concomitant incontinence operation at the time of prolapse repair, they will most likely exhibit significant postoperative urinary incontinence.
As reported by Wall et al. (1994), simple office bladder filling has a high positive predictive value for urodynamic SUI when compared with multichannel urodynamic testing. We perform residual urine determination, simple bladder filling, and stress testing with a full bladder in prolapse patients. The patient is asked to perform serial coughing in both the standing and supine positions. Observation includes the presence or absence of immediate, nonsustained urine leakage. We then use a rectal pledget, which gently reduces the prolapse to straighten the urethra. Care is taken not to press on the anterior wall and induce artifact. The presence or absence of leakage is again noted in both the supine and standing positions. A standing rectovaginal examination may be considered to assess for enterocele descent. If the patient demonstrates projectile, immediate, and nonsustained leakage with cough, we perform a urethropexy at the time of vaginal vault surgery.
Discussant: John R. Miklos
Preoperative Preparation
After the patient has undergone an evaluation, she should be presented with her diagnosis and potential recommendations. She should be informed that her condition is not life threatening and that only she can make the decision whether her condition is severe enough to warrant surgical correction. The patient should be presented with both the nonsurgical and surgical options. She should be offered a pessary, and if she declines she should then be offered the option of surgery. The surgical technique, cure rate, risks, and benefits of the operation should be discussed. If the patient elects to have surgery, she should have a preoperative chest X-ray, electrocardiogram (ECG), and necessary blood work. She would be instructed on a 48-hour bowel prep before surgical intervention: begin a full liquid diet 48 hours before surgery, followed by a clear liquid diet 24 hours before surgery and a one-half bottle of magnesium citrate. She can have nothing by mouth after midnight before the day of surgery.