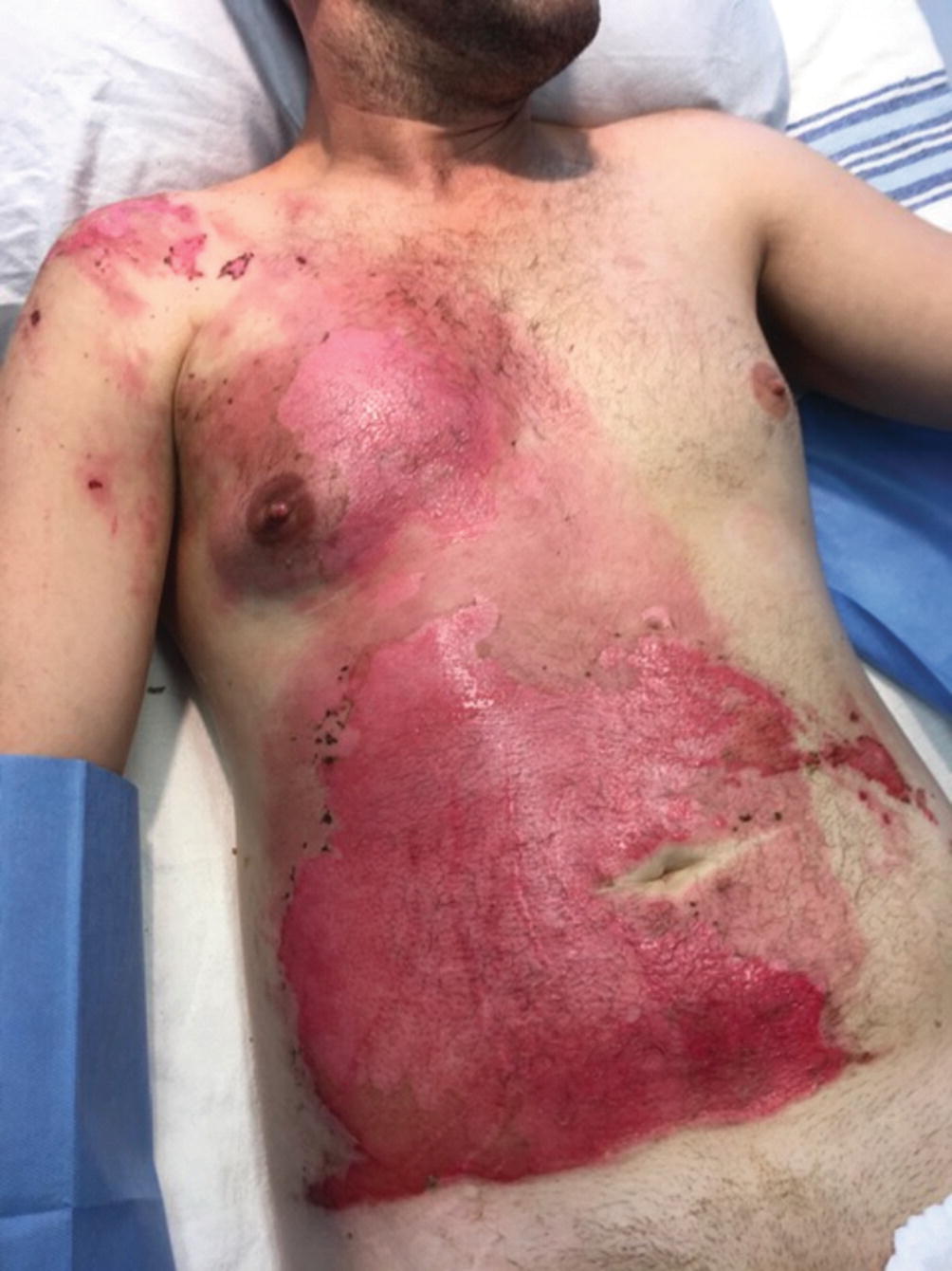
Sulfuric acid upper extremity burn
- 3.
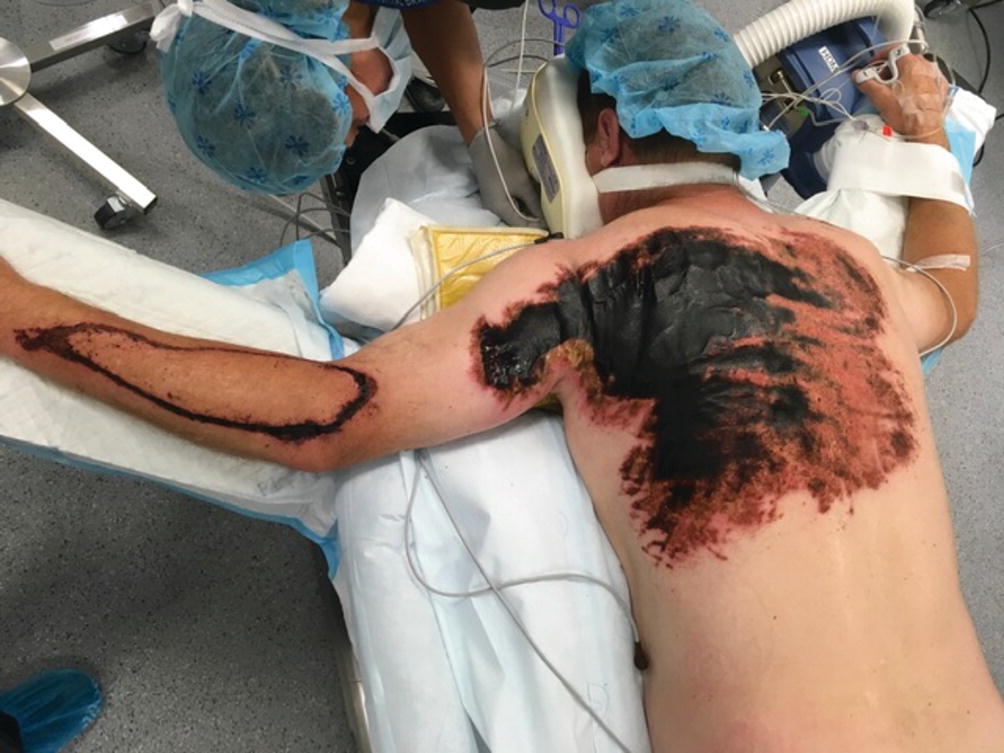
Phosphorus
Burns caused by phosphorus occur secondary to reacting with the oxygen and its derivatives. It is a frequent agent in wars and acts of terrorism. The injury continues as long as the agent is in contact with skin; therefore, it is imperative to remove the offending agent from skin using metal forceps. Use of Woods lamp could be beneficial in identifying the particles. The antidote for treatment of phosphorus contact is lavage with 1–2% copper sulfate. Excess copper sulfate must be removed, because absorption may cause hepatic and renal damage [19]. The systemic effect of phosphorus absorption could cause serious alterations in the concentration of body’s phosphorus and calcium, leading to arrhythmias together with hemolysis, hematuria, and hemoglobinuria. This can cause kidney failure and even death. Therefore, alterations in EKG after exposure to phosphorus is important and the best course of action is to wash off the body with copious amount of soapy water immediately after the accident [2, 23, 24].
Phosphorus burn
40.5.2 Alkaline Agents
- 1.
Cements
Burns secondary to building cements usually do not have much pain. This is compared to hydroxides. The exact mechanism of burns secondary to cements is not clear. These compounds usually contain multiple metals such as chrome. Thus, a combination of high alkaline pH, contact, and allergic dermatitis could be in play [25].
- 2.
Hydroxides
These chemicals have great penetrating and destructive potential. They usually cause extensive soft tissue damage until the chemical is inactivated and the pH is neutralized. Use of weak acid solutions such as 1% acetic acid has been proposed in some studies as neutralizing method of treating these injuries. One should always be considered of the exothermic reaction that these could cause. Copious irrigation with water until pH normalizes is also recommended. One exception to this is calcium hydroxide. This chemical has a very high hygroscopicity and causes a very exothermic reaction with water that could cause even a more severe injury [26].
Sodium hydroxide burn
- 3.
Organic solutions
These materials act as dissolving agents for the lipid membrane of cells and causing the disruption of the cellular protein structure.
Injuries due to these chemicals (petrol, kerosene, cresol, phenol, etc.) are as a result of direct contact with skin. Gasoline in a prolonged direct contact with skin can lead to full-thickness burns [27]. Its systemic effect could lead to paralysis, coma, hepatic damage, or motor-sensory neuropathies. Pulmonary lesions are the result of the chemical changing the production of surfactant. These organic materials could easily pass to the blood stream and cause cardiac or renal dysfunctions and even in severe cases, multiorgan failures. Kerosene is a lipid solvent and copious irrigation with soap is recommended to remove the offending material. Phenol and cresol on the other hand cause coagulation necrosis [28]. Following the exposure, dark thick scabs could form with minimal edema. The systemic effect could vary from hematuria and renal damage, digestive hemorrhages, increased liver transaminase with hepatic injuries, anemia, dyspnea, and hypotension.
- 4.
Inorganic solutions
These chemicals damage the skin by direct binding and formation of salts. Many of these reactions are exothermic leading to thermal injuries.
40.6 Prevention
From a preventive point of view, proper legislations, and work-related regulations and trainings are mandatory to decrease these injuries. Compliance with safety regulations such as proper outfit, suitable clothing, boots, gloves, and protective goggles should be mandated. A product sheet should be available and correct labelling should be placed. Prevention is essential and in order to limit the damage, water points and showers should be placed next to the hazardous areas. Immediate washing and communication with the medical professionals upon transfer of the patient for proper treatment is recommended.
Summary Box
Chemical burns are caused by corrosive materials to the skin or mucosa
There are six mechanisms of injury after chemical burns: reductive, oxidative, corrosive, protoplasmic poisons, vesicants, and desiccants
Upon these injuries, patients should be treated through the ATLS guidelines with specific attention and modifications according to the type of chemical
Specific organ systems involved need to be treated differently
Ocular injuries are medical emergencies and should be treated emergently according to the injury grading
Inducing vomit or charcoal/neutralizing agents are not recommended for treatment of ingestion of chemical
Acids lead to more frequent renal issues than alkaline
Knowledge for treatment of specific chemical types in both acidic and alkaline burns are necessary
Prevention is the main step in reducing the incidence of these injuries
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








