Breast Reconstruction with Form-Stable Implants
Maurizio Nava
Angela Pennati
Andrea Spano
Giuseppe Catanuto
Historical Background
Breast augmentation with the use of silicone gel breast implants was developed by the physicians Thomas Cronin and Frank Gerow in conjunction with Dow Corning and was first performed in 1962. Silicone gel implants have been further improved, and between the 1960s and the 1980s alone, three new generations appeared. The modifications concerned mostly the shell thickness and the viscosity of the gel.
The first generation of breast implants, used from 1963 until 1972, was characterized by both thick gels and thick shells. The characteristics of the second generation of implants were exactly the opposite; these implants were used from 1972 until 1980. The 1980s brought further improvements: increase of gel cohesiveness, thicker shells, and the introduction of a protective barrier layer to reduce the eventual diffusion of the gel through the implant.
In 1993, Inamed Corp. (formerly McGhan Medical Corp.) introduced to the market its style 410 implant, considered to be the fourth generation of breast prosthesis. The major difference with respect to the third generation consisted of a filling with a much more cohesive and firm silicone gel. The introduction of a denser and more form-stable gel in style 410 implants was done to avoid its eventual migration to the surrounding area in case of shell rupture. In fact, it has been shown that the gel filling of style 410 breast implants remains inside the prosthesis even after trauma.
In 2001, a new type of implant, with a 9-cell soft-touch matrix, was developed. The cross-linking of gel molecules was relatively loose, resulting in a softer texture than that of the style 410 implant. “Memory” and shape conservation in the standing position, however, were significantly lower. That is why in 2002 the matrix was modified to 12 cells by adding a fourth column and therefore providing an extra projection.
Another important benefit of introducing a cohesive gel is its capacity to maintain a proper, proportionate shape. It gives the possibility to restore a natural breast contour and to achieve upper pole fullness.
In 2004, Inamed developed a new breast implant, style 510 dual gel, an innovative product with a unique dual gel combination. The posterior part of the implant is filled with the standard cohesive silicone gel like the one of the style 410; the anterior part is filled with a special high-cohesive gel. This feature provides more projection and support of the nipple-areola area.
The shape of the implant is based on that of the style 410 XP, presenting the same dimensions in height and width. It differs, however, from the previous model in its posterior wall, which is concave, and the implant’s edges are thinner.
Aim of Extra-Projection, Implant-Based Reconstructive Strategy
Breast reconstructions were planned in the past with the purpose of rebuilding an identical and possibly symmetric breast mound. Thus, large and sagging glands were replaced by autologous flaps, while reconstructions with implants were indicated mainly for small and medium-sized breasts with a moderate degree of ptosis. Operations on the healthy breast in search of symmetry were considered undesirable because of undue scarring (1,2). Extra-projection form-stable devices gave us the chance to modify this problem by saving women from the severe biomechanical complications of myocutaneous flaps (3,4,5,6). This reconstructive strategy aims at the creation of a bilateral, cosmetically effective, medium-size breast (between 400 and 500 cc) for all women rather than having a ptotic one exactly matching the contralateral breasts. Adjustments on the healthy side are part of this plan.
Molding the opposite breast to the reconstructed one in our opinion allows for:
Reduction of surgical aggressiveness
Standardization of procedures (simplified implant selection)
Increase in patient satisfaction with final result very close to cosmetic surgery
Indications and Contraindications
Patients diagnosed with early-stage breast cancer and scheduled to undergo mastectomy and reconstruction can be dealt with by this technique, irrespective of their mammary shape and dimensions. Women who decide to have a delayed reconstruction are also considered eligible. Previous radiation treatment (either after breast conservative surgery or mastectomy) used to be considered an absolute contraindication to this kind of breast reconstruction (7,8). Modern technologies based on autologous fat transplantation offer the possibility to overcome this obstacle (9).
Operations on the healthy breast are part of the form-stable implant–based breast reconstructions. Although we are aware of several concerns about a higher risk of contralateral malignancies in women who have already had breast cancer on one side, contralateral adjustments are not contraindicated in our experience.
We acknowledge that evidence provided in the literature regarding contralateral augmentations in these settings is still debatable. However, in healthy women it is well known that despite the diminished sensitivity of mammography with implants,
augmented and nonaugmented patients are diagnosed at a similar stage and have a comparable prognosis (10). The increased role of magnetic resonance imaging will probably support effective follow-ups (11).
augmented and nonaugmented patients are diagnosed at a similar stage and have a comparable prognosis (10). The increased role of magnetic resonance imaging will probably support effective follow-ups (11).
Magnetic resonance can similarly solve doubts that were raised in the past for marked scarring after breast reduction of the unaffected gland. Reduction surgery could be beneficial on the healthy side even from an oncologic point of view. Petit et al. (4), for example, reported that in 4.6% of patients who received a contralateral reduction an occult synchronous carcinoma was revealed in the healthy breast.
Preoperative Planning
One of the main limitations of implant-based breast reconstruction is the higher complication rate when radiotherapy is required or has been employed (7,8). In mastectomies after breast conservation and radiation, we usually advise flap-based reconstructions. These are commonly accomplished in our unit using microvascular deep inferior epigastric artery perforator flaps to preserve large muscular islands.
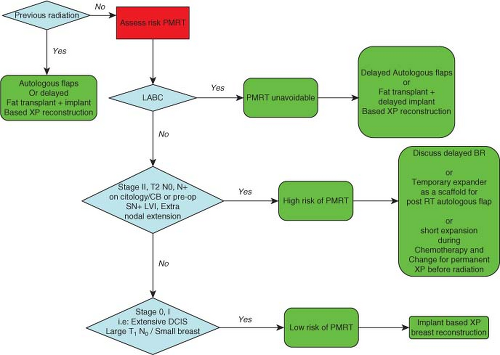
Preoperative identification of patients at high risk of postmastectomy radiotherapy is not very easy. Women affected by locally advanced breast cancer (LABC), in which radiation is mandatory, are normally candidates for a delayed flap-based reconstruction. Alternatively, a delayed implant-based two-stage breast reconstruction can be performed after treatment of skin radioinduced damage with adipose-derived stem cells. In demanding patients, a temporary expander can be placed soon after mastectomy, and radiotherapy can be subsequently administered. We do not recommend this option due to the high complication rate and the possible reduction of treatment efficacy (delayed irradiation due to complications, bundle hampering by metal plate on device, etc.). The final reconstruction requires autologous flaps or permanent implants followed by treatment of radioinduced damage with adipose-derived adult stem cells (9,12) (Fig. 36.1).
Women affected by stage II breast cancer (clinical, positive premastectomy sentinel node biopsy, or ultrasound-guided nodal cytology/core biopsy), tumors with high proportion of positive lymph nodes, lymphovascular invasion, or extranodal invasion can be considered at high risk of extensive nodal involvement (13). In such cases, a delayed breast reconstruction can be discussed with the patients and can be performed either with flaps or implants after adipose tissue transplantation if radiotherapy has been required.
When chemotherapy has not been given preoperatively, tissue expansion can be performed during systemic treatment and a permanent prosthesis can be inserted before radiation treatment commences (14). This strategy is being tested in our
institution, yielding promising preliminary results. In patients who have already had neoadjuvant chemotherapy and are willing to undergo immediate breast reconstruction, a temporary tissue expander can be inserted. If the final histologic report demonstrates extensive nodal involvement, then radiation has to be administered with the device in place, and the reconstruction process will proceed as described for LABC.
institution, yielding promising preliminary results. In patients who have already had neoadjuvant chemotherapy and are willing to undergo immediate breast reconstruction, a temporary tissue expander can be inserted. If the final histologic report demonstrates extensive nodal involvement, then radiation has to be administered with the device in place, and the reconstruction process will proceed as described for LABC.
Two-stage extra-projection implant–based breast reconstruction can be safely performed in all women at low risk of nodal involvement such as those affected by stage 0/I disease (extensive ductal carcinoma in situ, T1 N0 in small breasts, clinical N0, negative preoperative sentinel node biopsy).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree