10
Breast cancer
treatment of uncommon diseases
Pregnancy-associated breast cancer
Pregnancy-associated breast cancer (PABC) includes breast cancer diagnosed during pregnancy, up to 1 year after delivery, or during lactation. PABC is rare; it constitutes 0.2–3.8% of all breast cancers, occurring in 1 in 10 000 to 1 in 3000 pregnancies.1 Nonetheless, PABC is the second most common malignancy in pregnant women (cervical cancer is the most common). As more women delay childbearing, the incidence of PABC may increase.2
Age for age, compared to non-pregnant women with breast cancer, pregnant women with breast cancer tend to be diagnosed at later stages.3 The aetiology of this phenomenon is unclear. One possible explanation is that PABC may have a more aggressive biology. Alternatively, the significant anatomical and physiological changes in the breast during pregnancy may delay diagnosis.
Pathology
Histologically, PABC and non-PABC are similar. Compared to non-PABC, PABC tends to be oestrogen- and progesterone-receptor negative, while data on expression of human epidermal growth factor receptor (HER-2)/neu are conflicting.4,5 PABC stains strongly for Ki67 and p53; the clinical significance of these findings is undetermined.6
Clinical presentation
The majority of PABCs present as a palpable breast mass. Gravid breasts undergo significant ductal and lobular proliferation under the influence of elevated levels of oestrogen, progesterone, prolactin and chorionic gonadotrophin. Mammary blood flow increases by 180%; the breast may become nodular and double in weight and volume.7 Therefore, clinical breast examinations may be difficult, and may contribute to a delay in diagnosis and consequential poorer prognosis. Moreover, the differential diagnosis of breast mass in pregnant women is broad, including lactating adenoma, fibroadenoma, cystic disease, lobular hyperplasia, galactocoele, abscess, lipoma, hamartoma and PABC; 70–80% of breast biopsies in pregnant women are benign.8
Because PABC is rare, detailed evaluations of risk factors for PABC development are lacking. One study of 383 patients, including 192 patients with PABC, found that patients with PABC were 300% more likely to have a family history of breast cancer than age-matched, non-pregnant, non-lactating counterparts.3
Diagnosis
As in all women, dominant breast masses in pregnant women should be investigated with imaging and biopsies. While screening mammography is not routine for pregnant women, diagnostic mammography may be useful and can be performed safely with the use of abdominal shielding. Despite the fact that women with PABC are young and have dense, proliferative breasts, diagnostic mammograms detect 78–90% of palpable PABCs.9
However, core-needle biopsy is not without risk. In addition to bleeding, milk fistulas are known rare complications.10 Therefore, core-needle biopsy ought to be used with caution for the diagnoses of centrally located lesions in lactating women.
Metastatic work-up for non-PABC and PABC are similar and are guided by clinical stage and constellation of symptoms. Chest radiography with abdominal shielding is safe. Computed tomography (CT) scans are generally discouraged due to high radiation exposure. While magnetic resonance imaging (MRI) does not utilise ionising radiation – and, in fact, has been utilised in foetal imaging in utero – gadolinium is known to cross the placenta and to affect foetuses adversely in animals and is not recommended.5,11 No studies have investigated the role of position emission tomography (PET) in pregnant patients; in fact, pregnancy is often an exclusion criterion. Bone scans using lower doses of radioisotope have been performed in pregnant women, but are not generally recommended.11
Treatment (Fig. 10.1)
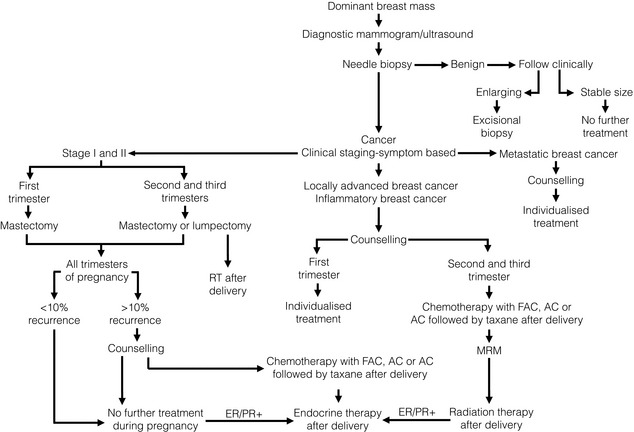
Figure 10.1 Management algorithm for the pregnant woman with a dominant breast mass. ER/PR+, oestrogen and progesterone receptor positive; FAC, fluorouracil, doxorubicin and cyclophosphamide; MRM, Modified Radical Mastectomy.
A landmark report of 2565 patients demonstrated no differences in congenital abnormalities between pregnant women who did and did not undergo surgery.12 However, surgery during pregnancy was associated with an increased rate of spontaneous abortion. The risk of spontaneous abortion is highest in the first trimester, and the patient and surgeon may choose to defer surgery until after the 12th week of pregnancy.
Axillary staging
Previously, sentinel lymph node biopsy (SLNB) was not recommended in gravid or lactating women. Lymphazurin blue, patent blue V and methylene blue, the most commonly used dyes, have not been studied in pregnant women and their safety has not been established. Technetium-99 m, the most commonly used radioactive tracer, involves low doses of radiation (1.85–3.7 MBq). Gentilini et al. found that sentinel lymph nodes were successfully identified in all patients, foetal radiation exposure was minimal and there were no adverse foetal outcomes.13 Although there are no medical contraindications to lymphatic mapping with radioactivity during pregnancy, it remains to be seen if this procedure will be widely adopted.
Systemic therapy
In general, chemotherapy is contraindicated during the first trimester, for it exposes the foetus to an increased risk of abortion and malformation. In a small series, Ebert et al. noted that all the abortions that occurred in women during pregnancy were in those women who had received chemotherapy during the first trimester of pregnancy; while chemotherapy during the first trimester resulted in a 14–19% risk of foetal malformation, chemotherapy during the second trimester resulted in a 1.3% risk.14 A prospective cohort study of 24 women, who received 5-fluorouracil, doxorubicin and cyclophosphamide during the second and third trimesters, found no congenital malformations, or short- or long-term complications in the infants; there were no stillbirths, miscarriages or perinatal deaths. Except for one child with Down’s syndrome, the vast majority of infants demonstrated ‘normal development’ and eventually grew into functional school-aged children.15,16
During pregnancy, methotrexate should be avoided due to a risk of associated abnormalities. A review examining the use of taxanes, vinorelbine and trastuzumab demonstrated that taxanes and vinorelbine were safe for mother and offspring in the limited experience available, but trastuzumab was associated with anhydramnios in 50% of cases.17 Endocrine therapy is not recommended during pregnancy. Tamoxifen has been associated with spontaneous abortions, teratogenicity and foetal demise.18
In general, for women with PABC with positive lymph nodes, chemotherapy is ideally administered after the first trimester, but within 6 weeks of surgery. For patients diagnosed early in the first trimester, this may be impossible. For women with PABC, negative lymph nodes and low-risk cancers (where the survival benefit of chemotherapy is 5% or less), treatment during pregnancy is usually avoided. For women with PABC also having negative lymph nodes and high-risk cancers, treatment decisions must be made on an individual basis. Chemotherapy ought to be stopped 3 weeks prior to delivery to avoid myelosuppression and septic complications in mothers and their offspring.6
Termination of and future pregnancy
Historically, the prognosis of PABC was considered so dismal that therapeutic abortion was advocated for all women. However, compared to women who terminated their pregnancies, women who continued their pregnancies did not demonstrate worsened overall or disease-free survivals.1,5 There are currently no formal recommendations for therapeutic abortions in patients with PABC. However, as discussed, therapeutic abortion can simplify treatment in patients with locally advanced and inflammatory breast cancers. Women should be counselled that chemotherapy may adversely affect future fertility.
Though fertility may be hindered by patient age and chemotherapy, retrospective studies demonstrated that pregnancy after diagnosis and treatment of breast cancer was safe. Gemignani et al. reported that compared to patients treated for PABC who did not become pregnant, those who did had equivalent or better survival,1 although this may be a result of selection bias. A population-based study conducted by the Danish Breast Cancer Cooperative Group found no difference in survival amongst 371 patients with PABC who became pregnant after treatment and 9865 patients with PABC who did not.19
Despite the fact that the rate of relapse is fairly constant for the first 10 years after treatment, some experts advocate waiting 2–5 years for future pregnancies,6 contending that this affords a period during which recurrences can become manifest – up to 2 years for high-risk cancers, up to 5 years for low-risk cancers. However, a population-based report showed that for women with localised disease and good prognosis, conception 6 months after completion of treatment was unlikely to reduce survival.20
Prognosis
Because of the anatomical and physiological changes of the gravid and lactating breast, PABC tends to be diagnosed at a more advanced stage. In one study, fewer than 20% were diagnosed prior to delivery, median tumour size at diagnosis was 3.5 cm, and 62% of PABC patients had lymph-node metastases compared to 39% of matched controls.1 Additionally, relative to non-PABC patients, PABC patients are more likely to have larger tumours, vascular invasion and distant metastasis.4
Historically, it was believed that, compared to non-PABC, PABC was intrinsically more aggressive and thereby carried a worse prognosis. However, recent studies have demonstrated this may not be correct. A review of 104 PABC and 564 non-PABC patients demonstrated that PABC had a more advanced T classification, N classification and stage.21 Moreover, the two groups did not demonstrate any differences in locoregional recurrence, distant metastases or overall survival. For patients with PABC, the timing of diagnosis and treatment was the only factor associated with overall survival; those who had prompt diagnosis and treatment had better overall survival than those who had delayed diagnosis and treatment. In an older study, Gemignani et al. found that, for stage I and stage II disease, PABC and non-PABC patients had similar survivals.1 However, compared to non-PABC, there was a trend towards a worse prognosis in stage III and stage IV PABC. Similarly, with negative lymph nodes, PABC patients had the same 5-year survival as non-PABC controls; however, for patients with positive lymph nodes, PABC and non-PABC patients had 5-year survivals of 47% and 59%, respectively.
Male breast cancer (Fig. 10.2)
In the 14th century, John of Arderne, an English surgeon, reported the first case of male breast cancer (MBC).22 He cared for a priest who had progressive nipple–areolar ulceration. Almost certainly he died of this disease.
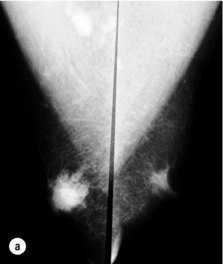
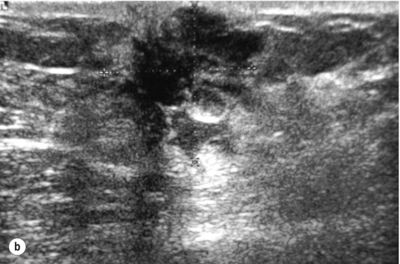
Figure 10.2 (a) Mammogram of male breast cancer in right breast. (b) Ultrasound of male breast cancer in right breast.
In the West, MBC accounts for less than 1% of all breast cancers and for 0.1% of cancer deaths in men.23 These rates have remained stable in the second half of the 20th century.24 However, rates of MBC have recently increased.25 Though a complete explanation is elusive, this is likely due in part to an increase in the number of men diagnosed with ductal carcinoma in situ (DCIS) between 1973 and 2001.26 Interestingly, there is an MBC ‘belt’ that extends from the Atlantic to the Indian Oceans in sub-Saharan Africa.27 In Tanzania, 6% of breast cancers are diagnosed in men.
Pathology
Almost all pathological types of breast cancer found in women have been described in men. Ninety per cent of MBCs are invasive; of these, 80% are ductal, 5% are papillary and 1% are lobular.28 The scarcity of lobular carcinoma probably reflects the paucity of terminal lobular units in male mammary tissue. Paget’s disease of the breast and inflammatory breast cancer are as frequent in men as they are in women.29 Less common subtypes, i.e. medullary, mucinous, squamous and tubular, have also been reported in men, but at lower frequencies than in women. Ten per cent of MBCs are non-invasive; almost all are DCIS,28 and most are of the papillary subtype and of low or intermediate grade.30 Lobular carcinoma in situ is rare.
When matched for age, stage and grade, men with breast cancer have a higher rate of oestrogen receptor positivity than women with breast cancer. A 25-year review of the Surveillance Epidemiology and End Results (SEER) database, which included 2357 MBCs, demonstrated oestrogen and progesterone positivity in 90% and 80% of breast cancers in men, and in 76% and 67% of breast cancers in women.31 Data on the overexpression of erbB-2 or HER-2/neu, p53, bcl-2, cyclin-D1 and epidermal growth factor receptor in MBC are limited, conflicting and inconclusive.
Clinical presentation
Though MBC can occur at any age, its incidence increases with age. The average age of diagnosis of breast cancer in men is approximately 10 years later than in women. Eighty-five per cent of MBCs present as a painless mass32 and most MBCs are centrally located, with up to 50% demonstrating nipple retraction, discharge, pain or ulceration.33 The time from the onset of symptoms to diagnosis is longer in men than in women – approximately 22 months. As a result, men often present at later stages.34
There are multiple risk factors for the development of MBC. Hormonal misbalance is one of these. Men with a history of undescended testes, congenital inguinal hernia, orchiectomy, orchitis, testicular injury, infertility, Klinefelter’s syndrome, and obesity and cirrhosis (both of which induce a hyper-oestrogenic milieu) are at elevated risk.35,36 In particular, Klinefelter’s syndrome, which is characterised by a 47XXY karyotype, small testes and gynaecomastia, is associated with a 50-fold elevated risk.
Family history is another important risk factor. Between 15% and 20% of male patients with breast cancer have a family history of disease. The odds ratio for MBC increases with a positive family history and continues to increase as the number of affected first-degree relatives increases.37,38 These factors probably reflect an increasing risk of BRCA1 and BRCA2 mutations, which predispose both men and women to breast cancer. Previous reports have focused on the association between MBC and BRCA2 mutations. However, a recent report suggests that BRCA1 and BRCA2 carry equivalent elevations in risk.39 Male BRCA2 mutation carriers have a 6.3% cumulative risk of breast cancer.37,38 In the United States, 4% of MBCs are in BRCA2 mutation carriers; in Iceland, where a ‘founder effect’ is present, 40% of MBCs carry a mutation in BRCA2.37
As in women, radiation exposure is a risk factor for the development of breast cancer in men.36 One evaluation of a national database of 324 799 men without breast cancer and 121 men with breast cancer found that a history of a bone fracture and obesity were associated with the development of MBC; relative risk ratios were 2.2 and 1.79 respectively.40 Though gynaecomastia in and of itself was once thought to be a risk factor, it is no longer considered to be.34,36 Indeed, it may be the case that obesity is associated with both gynaecomastia and MBC.
Treatment
In a retrospective review, Scott-Conner et al. found significant differences in the treatment of matched men and women with breast cancer.41 Given anatomical considerations, compared to women, men are less likely to undergo breast-conserving surgery. Also, compared to women, men who undergo breast-conserving surgery are less likely to receive adjuvant radiotherapy. Men are also less likely to receive adjuvant chemotherapy.
Local therapy
Axillary staging guidelines are similar for men and for women. If axillary lymph nodes are clinically negative, SLNB should be performed. Sensitivity and specificity of SLNB in women and men are similar.42 Clinically positive nodes should undergo core biopsy or FNA for histological confirmation, which will allow axillary dissection without an SLNB.






