Fig. 16.1
Anatomy of the breast: 1 Chest wall, 2 Pectoralis muscles, 3 Lobules, 4 Nipple, 5 Areola, 6 Milk duct, 7 Fatty tissue, 8 Skin
16.2 Has Hyaluronic Acid Still a Role in Breast Augmentation?
The main advantage of using hyaluronic acid in breast augmentation is that the treatment is minimally invasive. In contrast, breast augmentation with permanent implants, such as silicon prosthesis, entails a surgical procedure under general anaesthesia. Additionally, the fact that the NASAH-based gel can be used under local anaesthesia results in a shorter recovery time than traditional surgical methods. Hyaluronic acid is degraded naturally and gradually, and therefore, some of the problems associated with permanent fillers, such as the permanency of technical errors or migration of the filler or prosthesis, are not applicable. Despite this, recent Q-Med’s decisions about Macrolane (Chap. 1.4) open a discussion about the role of this treatment in the future.
16.3 Macrolane for Breast Augmentation: Clinical Experience and Future Perspectives
Our experience in breast augmentation from July 2008 to April 2012 is described (case series of Doctor’s Equipe).
The management of complications arising from treatment and their relationship with injection technique will be analysed.
A total of 390 patients underwent breast augmentation with NASHA-based gel (Macrolane™ VRF 30) from July 2008 to April 2012 in our clinics in Italy. During this time, two groups of patients received NASHA-based gel injections using two different injection techniques. The first group (Group 1; n = 207) was treated from July 2008 to November 2009. In this group, multiple deposits of NASHA-based gel were injected through a single injection site at a level posterior to the mammary gland, without the use of ultrasonography guidance. Ultrasonography imaging was carried out on 46 patients, after an average time of 46 days post-injection to determine the location of NASHA-based gel in the breast. Active follow-up was not done on many patients of this group.
In the second group (Group 2; n = 183) treated from December 2009 to March 2012, NASHA-based gel was injected under ultrasonography guidance to ensure deposition in the intended location. A single deposit of NASHA-based gel into the space between the deep fascia of the breast and the pectoralis muscle was preferred, using a cannula to create a cavity with a uniform symmetrical globular shape. In the case of very small breasts, NASHA-based gel was injected into the space posterior to the pectoralis muscle (submuscular plane). Injection of NASHA-based gel into the space between the gland tissue and the deep fascia of the breast was avoided. This group underwent active follow-up for medical and echo graphic examination 1, 3, 6 months and 1 year after treatment. These examinations were carried out free of charge, to avoid losing patients to follow-up and to treat complications earlier.
16.4 Results
16.4.1 NASHA-Based Gel Injected Without Ultrasonography Guidance
A total of 207 women were treated with NASHA-based gel without ultrasonography guidance. At the time of analysis, follow-up had been carried out on 100 of the 207 women up to 1 year after treatment, and 72 patients (35 %) had received a second treatment with NASHA-based gel. None of the women treated at our clinic experienced severe complications that required surgical removal of the product. After injection of NASHA-based gel without ultrasonography guidance, a total of 47 % of patients experienced multiple lumps in the breast; however, in 92 % of cases, these lumps disappeared completely within 30 days of treatment. In seven patients, the lumps were removed by direct aspiration or hyaluronidase injection (see chapter: Complications of Macrolane). Baker 2 and 3 capsule formations developed in 10 of the treated breasts (2.4 %); seven of these breasts were treated with external manipulation (closed capsulotomy) and the other three breasts were treated with capsulotomies using a blunt cannula.
Ultrasonography analysis was performed on 46 patients (92 treated breasts) by our consultant radiologist after an average time of 46 days post-injection to determine the location of NASHA-based gel in the breast. In 43 breasts, NASHA-based gel was located in a space posterior to the deep fascia of the breast; in 35 of these breasts, NASHA-based gel was located between the deep fascia of the breast and pectoralis muscle; in three breasts, it was located in the pectoralis muscle; and in five breasts, it was located in the submuscular plane. There were no lumps or aesthetic problems in this group of patients and no one complained of pain or discomfort related to the NASHA-based gel injections. In the other 49 breasts, ultrasonography analysis revealed that the NASHA-based gel was located in a space superficial to the deep fascia of breast. These breasts showed a high rate of dislocation of NASHA-based gel into the breast parenchyma and into the subcutaneous tissue (12 breasts, 24 %) (Figs. 16.2, 16.3, 16.4, 16.5, 16.6,16.7).
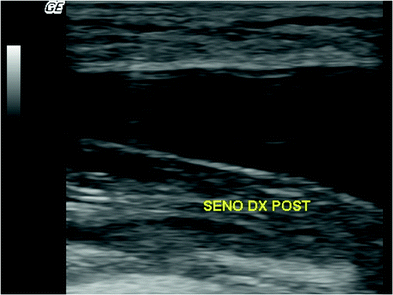
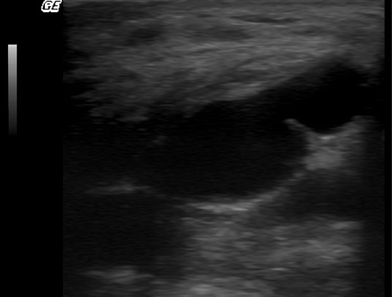
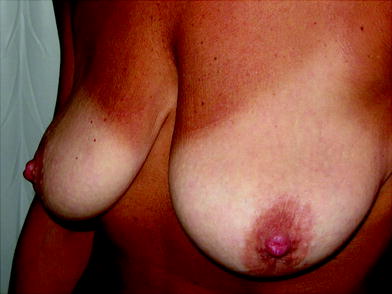
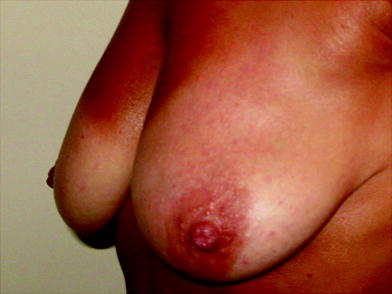
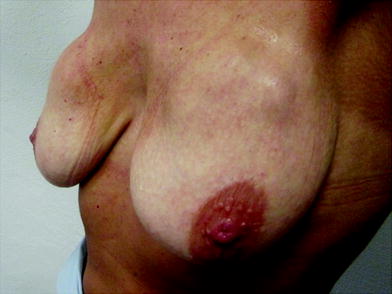
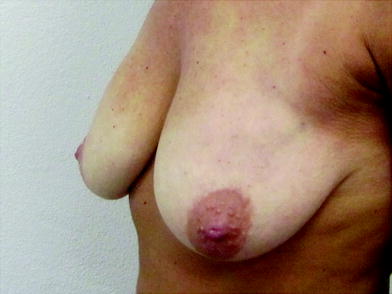

Fig. 16.2
Macrolane™ located superficially to the deep fascia


Fig. 16.4
Patient before treatment

Fig. 16.5
Patient 1 week after the treatment

Fig. 16.6
Patient 3 weeks after the treatment: big lumps can be observed

Fig. 16.7
After one month, the lumps are resolved
16.4.2 NASHA-Based Gel Injected Under Ultrasonography Guidance
A total of 183 women were treated with NASHA-based gel under ultrasonography guidance (Figs. 16.8, 16.1, 16.9, 16.10, 16.11, 16.12a, b, 16.13a, b, 16.14, 16.15, 16.16, 16.17, 16.18, 16.19, 16.20a, b, 16.21a, b, 16.22a, b, 16.23, 16.24, 16.25, 16.26, 16.27a, b, 16.28, 16.29a, b, 16.30a, b, 16.31a, b, 16.32, 16.33a, b, 16.34, 16.35, 16.36, 16.37, 16.38, 16.39a, b, 16.40, 16.41, 16.42




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








