Breast and Axillary Imaging for the Surgeon
Edgar D. Staren
Introduction
Dramatic and rapid change has become a common occurrence in all aspects of medicine; in few areas, however, has that change been as substantial and its implications as far reaching as in the subspecialty area of breast imaging for cancer. To put this in perspective, it is worth reflecting on the state of the art for diagnostic breast imaging in 1990, less than two decades ago. At that time, the superiority of standard roentgenographic mammography as compared with xeromammogram was being recognized. Rather than substantial attention being given toward optimizing the technique of choice, however, the bulwark of attention was focused on whether performance of mammogram of any type had value, particularly in the extremes of adult age groups. Interestingly, that debate continues even today for women 40 to 49 years of age. Also at that time, diagnostic ultrasound (US) of the breast was only rarely considered, and when performed, it was limited to straightforward differentiation of the cystic versus solid nature of a mammographically identified, nonpalpable breast lesion. Investigational techniques included scintimammography, thermography, magnetic resonance imaging (MRI), and others. Each of these has undergone significant evolution; despite that, with the possible exception of MRI, the role of scintimammography perhaps, certainly thermography, and others, remains ill defined. Evidently, therefore, the field of breast imaging remains a highly dynamic discipline.
One might rightly ask why after such a period of time a clear standard for breast imaging has not been delineated. As is the case in so many areas of medicine, the answer is simply dissatisfaction with the standard; until recently, that standard was mammography alone. Even today, conventional film screen mammography continues to be associated with an unacceptably high false-negative and false-positive rate; mammography sensitivity ranges from 66% to 91%, with specificity ranging from 88% to 96% (1,2). To put that into a more practical perspective, approximately 10% of patients undergoing a screening mammogram will be called back for a diagnostic mammogram. Approximately 1.5% (15 of 1,000) of those undergoing diagnostic studies will be referred for a biopsy; of these 15 referred for biopsy, approximately 30% or 5 of an original 1,000 will be found to have a cancer. Furthermore, at the other extreme, 20% of patients diagnosed to have a breast cancer will have had a negative mammogram within 1 year of their diagnosis (3). These results have caused investigators to persist on the search for the optimal method(s) to image the breast.
This review will address in some more detail those breast-imaging techniques deemed as “standard of care” and will also briefly consider a select number of those viewed as investigational and whose role is less well defined. Particularly for those in the former category (e.g., mammography, US, and to a lesser degree, MRI), it will focus on indications, technique, efficacy, and ongoing or anticipated updates.
“Standard of care” Techniques in breast imaging
Mammography
Historical Background of Mammography
Some of the earliest work on mammography actually had its origins in the early 1900s and was summarized in an article by Gershon-Cohen in 1938; in 1913, Albert Solomon, a surgeon, reportedly used a conventional x-ray machine to image cancers from 3,000 mastectomy specimens (4). Initial radiographs of the breast had numerous technical limitations that hindered their usefulness. They utilized industrial films rather than film receptors specific for mammography. This required high-energy radiation and, therefore, was associated with substantial radiation exposure. The equipment was not able to consistently compress the breast and used large focal spots. All of the mentioned factors caused substantial blurring of the image.
Despite these limitations, the potential benefit of mammography was being increasingly recognized. Raul Leborgne was credited with identifying the significance of punctuate calcifications demonstrated on mammogram as being indicative of breast cancer. He also emphasized the importance of compressing the breast tissue as one means of optimizing characterization of the calcifications (5). Radiologist Robert Egan was credited with paying particular attention to methods that optimized the technique associated with mammogram. For example, he introduced dedicated film for mammography so as to produce detailed images that were reproducible (6,7).
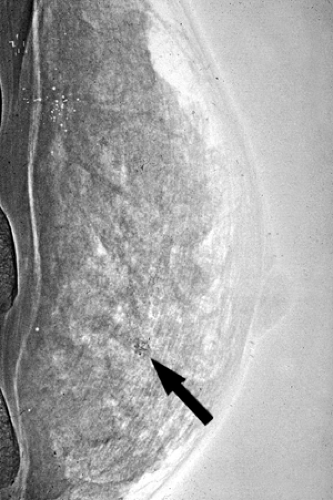
While these individuals acknowledged the potential value of screening mammogram and set about to prove its value in carefully performed studies, they do not appear to have considered the potential hazards of regular radiation exposure. Ultimately, this recognition plus the relatively poor quality of film-screen studies at that time encouraged the development of various technical modifications and most notably, xeromammography (Fig. 1.1). This technique involved a photoelectric method to record a roentgenographic image on a coated metal plate. It used low-energy photon beams and dry chemicals to provide the image. A report from Kalisher and Schaffer in 1975 demonstrated it to be quite accurate in detecting breast cancer. Moreover, it was more convenient and somewhat less expensive than film-screen techniques (8). These factors plus the nearly one-third radiation dose associated with xeromammography as compared with film-screen resulted in its rapidly gaining considerable favor.
Xeromammography was considered better at imaging calcifications while film-screen held the advantage in imaging subtle breast masses (Figs. 1.2 A,B and 1.3). Despite the inherent differences in image quality, the result was of only minimal clinical import as these differences were felt to be subtle. Gradually, however, advancements continued in film-screen technology such that the radiation dose utilized decreased to a point where it was becoming less than that utilized for xeromammography; this in turn reversed the movement back in favor of film-screen (8).
In 1969 Charles-Marie Gros reported that molybdenum used as an anode with a molybdenum filter produced low energy x-rays (9). About that same time, the first dedicated mammography unit was developed, utilizing molybdenum. A more specific x-ray spectrum and tube was incorporated so as to focus on the breast tissue. Subsequent advancements included reducing the time of exposure and improved ability to compress the breast safely. Tissue compression further lowered radiation exposure by minimizing
photon scatter, which would otherwise be absorbed. Ultimately, in the early 1980s, a reliable mechanical compression device facilitated the beginning of mass breast screening endeavors.
photon scatter, which would otherwise be absorbed. Ultimately, in the early 1980s, a reliable mechanical compression device facilitated the beginning of mass breast screening endeavors.
Screening Mammography
Indications and Efficacy
A number of reviews have examined the benefits associated with screening mammography (10). The positive predictive value of mammography for breast
cancer ranges from 20% in women younger than 50 years to 60% to 80% in women aged 50 to 69 years. Although trials have convincingly demonstrated a 30% reduction in breast cancer mortality in women 50 to 69 years of age who are screened annually or biennially, data on women under age 50 years are less clear. Criticisms of the mammographic screening trials and which limit strong conclusions for this group of women include the following:
cancer ranges from 20% in women younger than 50 years to 60% to 80% in women aged 50 to 69 years. Although trials have convincingly demonstrated a 30% reduction in breast cancer mortality in women 50 to 69 years of age who are screened annually or biennially, data on women under age 50 years are less clear. Criticisms of the mammographic screening trials and which limit strong conclusions for this group of women include the following:
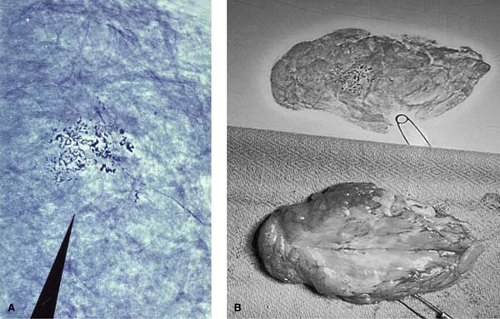 Figure 1.2 A. Magnified view of cluster of suspicious microcalcifications identified on screening xeromammogram noted in Figure 1.1. B. Lumpectomy specimen (lower frame) demonstrating an infiltrating carcinoma identified from a cluster of suspicious microcalcifications on screening mammogram (Fig. 1.1) and removal of same confirmed on specimen xeromammogram (upper frame). |
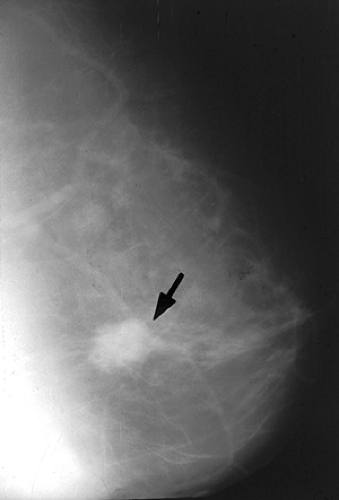 Figure 1.3 Film-screen mammogram (circa late 1970s) demonstrating an ill-defined mass lesion found to be an infiltrating carcinoma (arrow). |
Inadequately designed studies (e.g., failure of randomization and inadequate sample size),
Low compliance in the intervention group, and
High screening rates in the control group.
Following are among the well-reviewed trials evaluating screening mammograms:
HIP trial performed in the United States in 1963;
Malmo (1976), Two-County (1977), Stockholm (1981), and Goteborg trials (1982), all performed in Sweden;
Canadian trial (1980); and
Edinburgh trial (1978) performed in the United Kingdom.
Over several decades, these studies have been carefully scrutinized. Gotzche and Nielsen concluded that only two of these trials were randomized adequately (Malmo and Canadian) and that neither trial showed a reduction in mortality from screening mammography (12). They also noted that although the HIP, the Two-County, the Stockholm, and the Goteberg trials did show a reduced mortality associated with screening mammography, these studies were not optimally randomized. They did not include the Edinburgh trial in their analysis as it was considered to be biased. It is worth noting that although these trials examined nearly half a million women, they included few women older than 70 years and none younger than 39 years.
In a review by Armstrong et al. (13) for the American College of Physicians, they focused on screening mammography in women 40 to 49 years of age. They reviewed
117 other studies in addition to the above-mentioned 7 trials. They concluded that although the studies estimated a mortality risk reduction of 7% to 23% in this group of women, there was a substantial false-positive rate leading to a large number of unnecessary breast biopsies. In addition to unnecessary biopsies resulting in pain and surgical complications, other risks include radiation exposure and false-negative results. As such, it has been suggested that in women from 40 to 49 years of age, decisions regarding screening mammography should be guided by performance of periodic assessment of a patients risk for breast cancer (14,15).
117 other studies in addition to the above-mentioned 7 trials. They concluded that although the studies estimated a mortality risk reduction of 7% to 23% in this group of women, there was a substantial false-positive rate leading to a large number of unnecessary breast biopsies. In addition to unnecessary biopsies resulting in pain and surgical complications, other risks include radiation exposure and false-negative results. As such, it has been suggested that in women from 40 to 49 years of age, decisions regarding screening mammography should be guided by performance of periodic assessment of a patients risk for breast cancer (14,15).
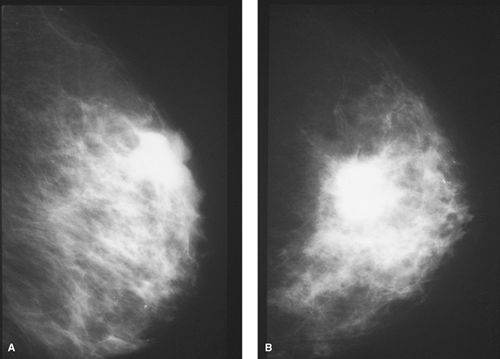 Figure 1.4 A. Mediolateral view film-screen mammogram of the left breast of a 38-year-old woman with a suspicious palpable mass shown on lumpectomy to be an infiltrating carcinoma. B. Craniocaudad view film-screen mammogram of the same breast as in Figure 1.4A. |
Recommendations
Most experts do agree that the risk of breast cancer for asymptomatic women younger than 35 years is not enough to warrant the risk of radiation exposure. In fact, most radiologists do not generally recommend performing screening mammography in women younger than 40 years. Exceptions, however, may include women at particular risk for breast cancer. That is, if there is a positive family history, BRCA positivity, or a suspicious palpable mass, mammography may still be important (Figs. 1.4 A,B).
The American Cancer Society, the American College of Radiology (ACR), and the American College of Obstetricians and Gynecologists recommend screening mammography for women aged 40 to 49 years every 1 to 2 years and annually after the age of 50 years (16,17). The American College of Physicians recommends biennial screening for women aged 50 to 74 years. The American Academy of Family Physicians, which recommends mammography screening for women older than 50 years, is currently updating its guidelines. The Canadian Task Force on the Periodic Health Examination recommends annual mammography for women aged 50 to 69 years and recommends against mammography screening for women aged 40 to 49 years (18). Similarly, the U.S. Preventive Services Task Force recommends mammography screening every 1 to 2 years for women aged 50 to 69 years (19).
Technique
When mammography is performed as a screening study, the intent of the examination is to detect changes in the breast in women who have no clinical signs of cancer. The
breast is placed in a dedicated mammographic machine gantry and compressed so as to even out the tissue and to maintain the breasts position. Screening studies involve performance of two views, mediolateral oblique and craniocaudad (Figs. 1.5 A,B). So as to decrease iatrogenic false-positive rates, women are discouraged from applying powder, deodorant, and even lotions before the study.
breast is placed in a dedicated mammographic machine gantry and compressed so as to even out the tissue and to maintain the breasts position. Screening studies involve performance of two views, mediolateral oblique and craniocaudad (Figs. 1.5 A,B). So as to decrease iatrogenic false-positive rates, women are discouraged from applying powder, deodorant, and even lotions before the study.
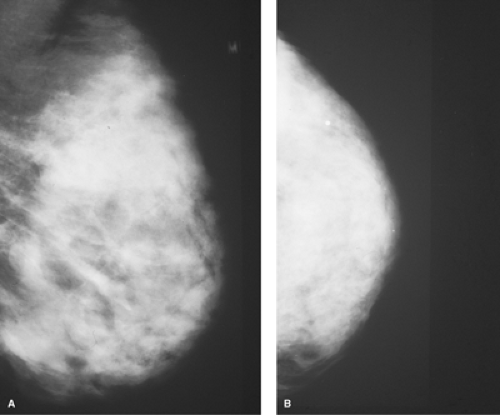 Figure 1.5 A. Film-screen mammogram of a diffusely dense parenchymal pattern breast in a left mediolateral oblique view. B. Film-screen mammogram of the same breast as in Figure 1.5A in a left craniocaudad view. |
As with any interpretative skill, mammography “reading” requires time and experience to develop. In reading a mammogram, it is useful to proceed with a two-step strategy. In the first step, one reviews the more prominent features of the image. This will involve a qualitative assessment of either normal anatomy or distortion thereof and with identification of obvious abnormalities such as mass lesions. During the second step, a more detailed and orderly review ensues, which aims for not only identification of more subtle features including calcifications but also more detailed analysis of lesions and associated findings such as parenchymal distortion, skin thickening, asymmetry.
Diagnostic Mammography
Technique and Indications
Diagnostic mammography is aimed at providing specific analysis of patients with abnormalities of the breast detected by clinical and/or screening mammographic examination. In addition to standard views, additional angles and special views may be required; special views may include magnification to enlarge an area of the breast that was shown to contain a possible mass or calcifications. Such views may also include spot compression used to press out areas of dense or distorted breast tissue and thereby better determine whether a lesion is present (Figs. 1.6 A,B). Patients may require additional studies such as US, MRI, in the course of the diagnostic evaluation. Indications for diagnostic mammogram include the following:
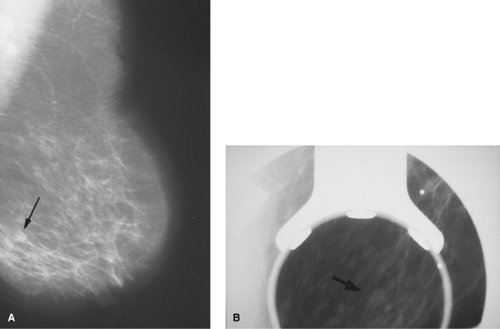 Figure 1.6 A. Mediolateral view film-screen mammogram demonstrating a less than 1 cm questionable nodular density (arrow) that led to recommendation for a diagnostic mammogram. B. Compression view diagnostic mammogram confirming nodular mass (arrow) demonstrated on screening mammogram in Figure 1.6A. |
A specific area of concern based on a suspicious breast-related symptom or sign (e.g., mass, axillary adenopathy, skin change),
Possible lesion or abnormality identified on screening mammogram,
Follow-up of a questionable area of concern,
Patients with implants, and
Patients who have been treated for breast cancer.
Digital Mammography
Although most studies are still performed with film-screen cassettes on a dedicated mammography gantry, there has been a recent migration toward performance of mammograms using digital detectors in a procedure referred to as full-field digital mammography (FFDM) (Figs. 1.7 A,B). First introduced by General Electric in 2000, digital mammography has been the most significant advancement in mammography in decades. With this technique, low-energy x-rays (25–40 kV) pass through the breast as with standard mammography but are recorded by an electronic digital detector rather than a film. Digital mammography offers several potential advantages over standard mammography, including the following:
Near-instantaneous imaging,
The ability to transmit the same image to various, even remote, locations, and
The ability to manipulate (e.g., reverse contrast, enlarge) the image so as to optimize visualization of abnormalities (Figs. 1.8 A–D).
The Digital Mammographic Screening Trial (DMIST) included nearly 50,000 women from across the United States and Canada who had no signs of breast cancer, had no history of breast cancer, were not pregnant, and did not have breast implants (2).
Among 42,760 women analyzed in a comparison of both film-screen and digital imaging, it was found that the latter was significantly advantageous in detecting breast cancers in three groups of women
Who were younger than 50 years,
Who had dense breasts, and
Who were premenopausal or perimenopausal (had their last period within a year of their mammograms).
 Figure 1.7 A. Screening digital mammogram of the right breast in mediolateral view demonstrating only scattered fibroglandular tissue but no abnormal calcifications or masses. B. Screening digital mammogram of same breast as in Figure 1.7A in a right craniocaudad view. |
Digital mammogram provided no benefit for women older than 50 years, those who did not have dense breasts, and those who were postmenopausal. These factors held true regardless of the race or risk for developing breast cancer.
Results
Mammographic Interpretation
Mammograms are optimally reported in a standard format that recognizes the importance of clear communication between the interpreting radiologist and the clinician. Such a format is the ACR Breast Imaging Reporting and Data System (BI-RADS) classification. The ACR BI-RADS format recognizes the inherent limitations associated with variation of fatty versus parenchymal tissue in the breast and separates breast imaging into categories based on these components as follows:
Almost entirely fatty—in which mammogram is very sensitive,
Scattered fibroglandular tissue—in which there may be a minor decrease in sensitivity,
Heterogenously dense tissue—in which there may be a mild decrease in sensitivity,
Extremely dense tissue—in which there may be a marked decrease in sensitivity.
Subsequent to this description of the overall breast composition, the bulk of the report attends to description of findings, final assessment, and recommendation. In describing findings, attention is given to masses, calcifications, and associated findings.
Masses are defined according to their location, size, shape, margin, and associated findings. Benign masses are characterized by round or oval shape and well-circumscribed margins. Malignant masses are characterized by irregular shape and architectural distortion as well as indistinct or irregular margins.
Calcifications are defined according to their location, distribution, size, and shape. Benign calcifications are characterized as being coarse, large, rod-like, rounded, egg-shell, or punctuate. They may also be characterized as typical of skin, vascular, or milk-of-calcium. Benign calcifications are often diffusely scattered or regional, whereas
malignant calcifications are more often grouped or segmental. Malignant calcifications are characterized as indistinct, pleomorphic, and branching or linear.
malignant calcifications are more often grouped or segmental. Malignant calcifications are characterized as indistinct, pleomorphic, and branching or linear.
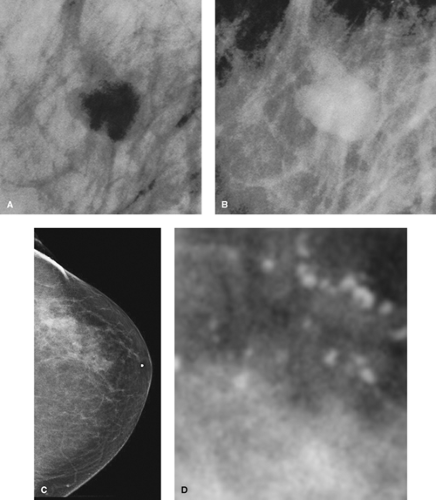 Figure 1.8 A & B. Smooth margined nodular density manipulated by digital mammography to demonstrate both zoom and reverse contrast capabilities. C. Diagnostic left breast digital mammogram craniocaudad view demonstrating suspicious upper outer quadrant density with spiculated appearance. D. Digital mammogram of density area from Figure 1.8C, manipulated with a zoom to show suspicious diffuse pleomorphic microcalcifications found on biopsy to be an infiltrating carcinoma. |
Associated findings consistent with a benign diagnosis include a skin lesion, whereas skin/nipple retraction, surrounding tissue architectural distortion, and prominent axillary adenopathy are suggestive of malignancy.
Based on these findings, an assessment is made and assigned to one of six categories. These categories indicate an associated recommendation, varying from category 0 (need additional imaging evaluation) to category 5 (highly suggestive of malignancy). Such an approach greatly facilitates communication with the clinician and as such, with the patient.
Category 0—need additional imaging evaluation.
Category 1—negative; recommendation: routine screening mammography.
Category 2—benign finding; recommendation: routine screening mammography.
Category 3—probably benign finding; recommendation: follow-up in x (short-interval) months.
Category 4—suspicious abnormality; recommendation: biopsy should be considered.
Category 5—highly suggestive of malignancy; biopsy is strongly recommended.
Breast US
Indications
Ultrasound refers to any frequency of sound greater than 20 KHz, that is, the frequency above which humans cannot normally hear. All sound travels through different tissues at different rates of speed. When sound hits the interface between two tissues with different effects on the speed of sound, an echo is created. Medical, and in this case, breast US, produces visual images based on echoes that occur at such interfaces (20).
As with mammography, the role of US in the evaluation of breast disease continues to evolve. Historically, US was used to differentiate the cystic versus solid nature of mammographically identified, nonpalpable breast lesions (Fig. 1.9). Even cysts as small as a few millimeters in size may be diagnosed under proper circumstances. If a cyst is demonstrated, then further mammographic workup may be avoided (21). Increasing quality of US equipment beginning in the early 1990s included availability of high-frequency and multifrequency linear array transducers and computer-enhanced imaging, all of which led to increased quality of breast US images (22,23). Improved imaging has allowed for better delineation of a number of US characteristics of breast lesions; this in turn facilitated their categorization into groups based on the increasing likelihood of benign versus malignant (24,25,26). The improved quality of US equipment has expanded indications for breast US to include evaluation of a questionably palpable mass on physical examination associated with a negative or nonspecific mammogram (27) (Figs. 1.10 A,B




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree