Botulinum Toxin
Doris M. Hexsel
Camile L. Hexsel
Leticia T. Brunetto
The value of facial and corporal aesthetic image plays an important role in society and has grown with the development of numerous minimally invasive procedures. Patients are looking for safe, new, and less invasive techniques for facial rejuvenation as they search for quick results with low incidence of side effects. Certain minimally invasive procedures are also practical, as patients can immediately return to their activities.
Repeated facial movements, caused by contraction of the muscles of facial expression, are one of the most important etiologic factors of facial expression lines and rhytides. Such repeated movements also play a role in the development of redundant skin around these lines and rhytides. Botulinum toxin (BT) injections represent one of the latest and most revolutionary treatments in facial rejuvenation, targeting hyperkinetic muscles of facial expression. They are currently considered the best alternative for the treatment of dynamic wrinkles.1 BT can be used alone or in combination with other minimally invasive or invasive techniques.2,3,4
In the last decade, the results of the therapeutic and cosmetic use of BT types A and B have been published in many articles and chapters. The cosmetic use of BT has permitted better understanding of the physiopathology of the aging process, specifically of the role of muscular contraction in aging.
History of Clinical Use
In 1817, Justinus Kerner described botulism, a new disease characterized by muscle paralysis caused by the intake of poisoned food, probably spoiled sausage (from the Latin botulus, sausage).5,6
In 1897, Emile Pierre Van Ermengen isolated an anaerobic bacteria from contaminated food and injected the toxin of this bacteria, promoting disease in laboratory animals.6,7 He identified this anaerobic Gram-positive bacillus as the etiological agent of botulism and called it Bacillus botulinus.5,7 As bacillus identified aerobic organisms, in 1922, Bacillus botulinus received a new denomination of Clostridium botulinum, indicating its anaerobic nature.5,8 Clostridium botulinum produces botulinum toxin, the most potent neurotoxin known in nature.9 There are seven different serotypes of botulinum neurotoxins. Botulism in humans is most commonly caused by BT types A, B, and E.5
In 1949, it was demonstrated that BT inhibits the release of acetylcholine (Ach) from nerve endings.10
In 1973, Scott reported the use of BT in monkeys, demonstrating reversible ocular muscle paralysis during 3 months. In 1980, the toxin was introduced as a therapeutic agent when the same author published a study demonstrating BT injections as a successful adjunctive or alternative treatment to the surgical treatment of strabismus.8,11 After that, the clinical use of BT advanced to the treatment of other muscular disorders, characterized by excessive or inappropriate contraction, such as focal dystonia, blepharospasm, achalasia, anal spasm, vaginismus, and nystagmus.12,13
The cosmetic use of BT type A began in the late 1980s, when Carruthers and Carruthers noted an improvement in the glabellar lines after injections of BT for the treatment of patients with blepharospasm.14,15 At the beginning, only the upper third of the face was treated.16,17 Later, the cosmetic use advanced to applications in the mid- and lower face and neck.4,16,18,19,20,21 Other important uses in dermatology, such as hyperhidrosis22,23 and gingival smile,16 among others, have been developed in recent years.
The therapeutic use of BT for migraine and tension-type headache has also been described,16 and they include the treatment of some muscles of facial expression.
Available Botulinum Toxins
Seven neurotoxin serotypes were purified and identified. They are denominated as A to G. The serotype A has been shown to be the most potent. Currently not only the serotype A but also the serotype B are commercially available and authorized for therapeutic use.24 Only the serotype A is currently used for cosmetic purposes. Other serotypes are under investigation, as well as other toxins,17,25,26,27,28 and perhaps new toxins will be available in the near future. The serotype B has shown to be an option for the treatment of patients resistant to serotype A.24,29
Botulinum toxin type A is available in three commercial preparations that are authorized for cosmetic use in some
countries, including Brazil: Botox (Allergan Inc., Irvine, California, USA), Dysport (Ipsen Limited, Paris, France), and Prosigne (Lanzhou Institute of Biological Products, China). Dysport is currently under investigation in the United States by the Food and Drug Administration (FDA), and its approval is expected for the next year. The commercialization of Dysport in the United States may go by the name of Reloxin. All these products produce similar and comparable effects in muscles and sweat glands, although they have distinct characteristics.2,16 Other BT type As are currently under investigation, such as Xeomin (Merz Pharmaceuticals, Frankfurt, Germany)30,31 and Neuronox (Meditox/Comedix, Seoul, South Korea).
countries, including Brazil: Botox (Allergan Inc., Irvine, California, USA), Dysport (Ipsen Limited, Paris, France), and Prosigne (Lanzhou Institute of Biological Products, China). Dysport is currently under investigation in the United States by the Food and Drug Administration (FDA), and its approval is expected for the next year. The commercialization of Dysport in the United States may go by the name of Reloxin. All these products produce similar and comparable effects in muscles and sweat glands, although they have distinct characteristics.2,16 Other BT type As are currently under investigation, such as Xeomin (Merz Pharmaceuticals, Frankfurt, Germany)30,31 and Neuronox (Meditox/Comedix, Seoul, South Korea).
Botulinum toxin type B is commercially available as Myobloc/NeuroBloc (Élan Pharmaceuticals Inc., South San Francisco, California, USA). Its use was approved by the FDA for the treatment of cervical dystonia in 200024,32 and was investigated in patients who have developed antibodies to type A.29,33 Studies have shown the safety and efficacy of BT type B for the treatment of axillary hyperhidrosis,34,35 although it is not currently approved for cosmetic use.32 The data in the literature evaluating the safety and efficacy of BT type B for the treatment of facial wrinkles is sparse.32
Table 22-1 Characteristics of Botox and Reloxin | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Mechanism of Action
Botulinum A exotoxin prevents the release of Ach from the presynaptic neuron of the neuromuscular junction of striated muscles, producing chemical denervation and consequently reversible paralysis of the muscles.16,25,26,36,37
There are three steps involved in the inhibition of the release of neurotransmitter Ach from the presynaptic nerve terminal at the neuromuscular junction:37,38
Binding: The toxin binds to a receptor at the surface of the cell membrane of the presynaptic neuron.
Internalization: The toxin is internalized within the neuron.
Blockage: The release of Ach-containing vesicles is blocked by the interaction of the toxin with intracellular proteins that are responsible for vesicle release.
Weakness of affected muscles begins 6 to 36 hours after injections of BT, depending on the dose,26 and is clinically evident 3 to 7 days after treatment, rarely later. This action lasts for 3 to 6 months, when the formation of new neuromuscular junctions (neurogenesis) occurs, permitting muscular function.5,16,25,37,39,40,41
Pharmacology, Conservation, Dilution, and Storage
Botox, Reloxin, and Prosigne are marketed in a vacuum-dried lyophilized form, but only Botox and Prosigne are vacuum sealed.43,44 Any vial of Botox or Prosigne45 without vacuum seal should be discarded.43 The expiration date indicated on the package of all these toxins should be respected.43,44,45,46
Myobloc is provided as a clear and colorless to light yellow sterile injectable solution in 3.5 mL glass vials.46
The particular characteristics of Botox and Reloxin are shown in Table 22-1.
Prosigne is made with Clostridium botulinum toxin type A hemagglutinin complex, 5 mg gelatin, 25 mg dextran, and 25 mg sacarose.45
Myobloc is made with Clostridium botulinum toxin type B in 0.05% human serum albumin, 0.01 M sodium succinate, and 0.1 M sodium chloride at an approximately pH 5.6. The neurotoxin complex is recovered from the fermentation process and purified through a series of precipitation and chromatography steps.46 Myobloc vials contain 5,000 units (U) of BT type B per milliliter.46 It is available in three vial presentations of 2,500, 5,000, and 10,000 U.47
The vials of Botox and Prosigne contain 100 units of BT type A.43,45 The vials of Reloxin contains 500 units of BT type A.44
The storage temperature recommended by the manufacturers are 2°C to 8°C (35°–45°F) for Reloxin,44 Prosigne,45 and Myobloc46 and -5°C (25°F) for Botox.43
Myobloc has shown stability for months when stored appropriately at this temperature.48 Its manufacturer recommends to avoid freezing or shaking the vial of Myobloc.46
The recommended therapeutic doses of Botox and Reloxin are different,49 and there are divergences about their equivalence.50 Some publications consider that 1 U of Botox is equivalent to 3 U to 5 U of Reloxin.21,50,51,52,53 The equivalence ratio use is 1:2.5 U between Botox and Reloxin, and similar results can be achieved. A recent pilot study performed by two of the present authors (Hexsel and Hexsel) demonstrated similar action halos between Botox and Reloxin at a conversion ratio of 1:2.5 U when injections are performed using the same volume and at the same depth on frontal muscles.54 It is important to emphasize that side effects are more frequent when higher conversion rates, such as 1:3 or 1:4 or more, between Botox and Reloxin are used.53,55
Botox and Prosigne have 100 units of BT type A per vial and seem to be equivalent, although there is currently no evidence of this equivalence in the literature.
Manufacturers of all BT type A recommend the reconstitution of their products, with 0.9% preservative-free saline solution.43,44,45,56,57
Myobloc does not require reconstitution.47 However, it also can be diluted with normal saline if necessary. Once diluted, the product must be used within 4 hours, as the formulation does not contain a preservative.46
Some studies tested the effects of the use of preservatives in BT type A dilutions.26,58,59,60 In 2002, a double-blind, randomized controlled trial demonstrated no difference in the efficacy of the treatment when comparing results of BT type A reconstituted using isotonic sodium chloride with and without preservative. This indicated that reconstitution with preserved saline solution does not impair the stability of the toxin.60 The use of BT type A reconstituted with preserved saline would also have additional advantages, such as longer periods of storage after reconstitution, reduced risk of bacterial contamination,26 and less painful when compared with preservative-free saline reconstitution.26,59,60
To date, only one fatal case of anaphylaxis was described in a patient who received Botox with lidocaine.61 Mixtures of BT with local anesthetics should be avoided because it may change the tertiary structure of the toxin and interfere with its pharmacokinetics.62
The dilution of Botox, Prosigne, or Reloxin may be performed with 1 mL, 2 mL, 5 mL, or 10 mL of preservative-free saline, aiming a concentration of 10 U/0.1 mL; 5 U/0.1mL; 2 U/0.1 mL; or 1 U/0.1 mL, respectively, for Botox and Prosigne or 50 U/0.1 mL, 25 U/0.1 mL, 10 U/0.1 mL, or 5 U/0.1 mL for Reloxin. Dilution of the commercially available toxins depends on the physician’s preference. For cosmetic purposes, dilution of the products with a smaller amount of volume is recommended because it results in higher concentration and allows the injection of smaller volumes. Additionally, it is safer and more precise, reducing the risk of complications and increasing the duration of the effects.5,16,17,27,41,63,64 To achieve a 1:2.5 U equivalence dose between Botox (or Prosigne) and Reloxin, an easy and practical way to go is to dilute Reloxin in double the amount of volume of saline used for Botox, which allows physicians to inject same exact volumes of both product for the same indications by same technique, reducing the possibility of conversion mistakes. This equivalence ratio allows the achievement of similar results regarding potency and duration of effects.54 However, proper to the high sensitivity of the muscles for BT, in some cases, especially at the lower face, the equivalence of Botox for Reloxin must be modified for 1:2 or lower.
The recommended doses of BT type A and B are different. The equivalence doses between BT type B (Myobloc) and BT type A (Botox) have been described as a conversion ratio of 50 U of Myobloc/1 U of Botox.65
BT type A may be inactivated by heat, freezing, shaking, excessive dilution, and surface tension generated from bubbles when the toxin is reconstituted.37
The reconstituted vial should be used within 4 hours according to the manufacturers’ instruction for Botox43 and Prosigne45 and 8 hours for Reloxin.44 They can be stored in the refrigerator at 2°C to 8°C. In 1996, a study demonstrated that reconstituted Botox refrigerated for up to 1 month had no substantial loss of potency.58 More recently, Hexsel et al. demonstrated in a multicenter, double-blind study that reconstituted Botox stored in the recommended conditions may be applied up to 6 weeks without losing its effectiveness.66 A similar study with Reloxin is being done, and the results will be available soon.
A comparative study using BT type A (Botox 5 U) and BT type B (Myobloc 500 U) for the treatment of forehead wrinkles described that BT type B produced a greater area of diffusion and a more rapid onset of action than type A.68
Indications of Botulinum Toxin Injections in Dark-skinned Patients
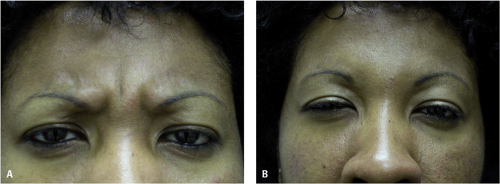
The cosmetic use of BT is recognized as an effective and efficient treatment for dynamic wrinkles, independently of sex, age, or race.18,69 Some characteristics that are unique to darker ethnic skin patients are relevant for BT injections in these individuals. They include the rounded facial phenotype, fuller upper and lower lips, and broader and flattened nostrils. Dark skin patients (DSPs) have fewer facial wrinkles than fair-skinned patients when comparing patients of similar age. Dynamic wrinkles in these individuals are also predominantly found in the upper face, especially in glabellar area (Fig. 22-1A).
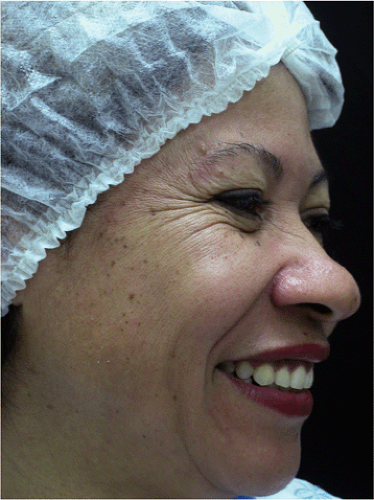 Figure 22-2 Periorbital (crow’s feet) wrinkles in a 50-year-old patient. She also recruits her nasal muscles when smiling. |
In the glabellar area, BT injections can erase or diminish lines70 and promote better position17,71,72 and shape of the brow (Fig. 22-1B).16,56,73,74,75 Repeated injections of BT can also prevent the physiologic brow ptosis.
Periorbital wrinkles are one of the earliest signs of the normal aging process. They are mainly caused by a combination of photodamage and muscular hyperactivity. Periorbital wrinkles appear after the age of 40 in DSPs and can be treated by injections of BT type A (Fig. 22-2).58,76,77 Nasal wrinkles can appear in these patients when smiling, but they are usually associated with excessive activity of glabellar muscles (Fig. 22-2).
Most DSPs present late physiologic blepharoptosis. However, eye bulging seems to be as frequent as in fair-skinned patients, because this condition is basically caused by anatomical configuration of the eyelid and subcutaneous fat.
Perioral injections of BT are rarely needed or performed in DSPs. BT can correct perioral wrinkles (Fig. 22-3), increase the fullness of the lips, and cause slight eversion of the upper lip.78 These effects are usually undesirable. Perioral wrinkles appear late in life and are rare in DSPs.
As the mentalis muscles can be very expressive in some DSPs, this is often an indication for BT injections for these individuals. The mentalis muscles have synergistic movements with the depressor angulis oris (DAO) muscles, so mentalis muscles relaxation determines less action of the DAO muscle.
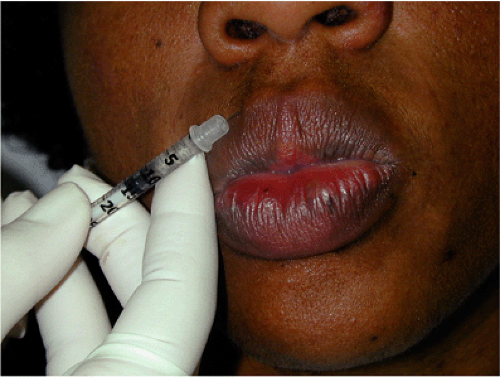 Figure 22-3 Small doses of BT injections can be used in the treatment of perioral wrinkles, such as 0.5 to 1 U of Botox or 1.25 to 2.5 U of Dysport/Reloxin. |
Aging neck is characterized by signs of photodamage, including wrinkles, laxity, loose skin, and poikiloderma of Civatte. These findings are more frequent in Caucasians and can be treated adjunctively by other techniques, such as surgical lift of the neck and tightening. Other signs of aging neck are wrinkles caused by the action of the platysmal bands. Selected cases in whom the platysmal bands are expressive can be successfully treated by BT type A applications.19,20,77,79 DSPs present fewer signs of photoaging, but the action of the platysmal bands seems to be similar to fair-skinned patients.
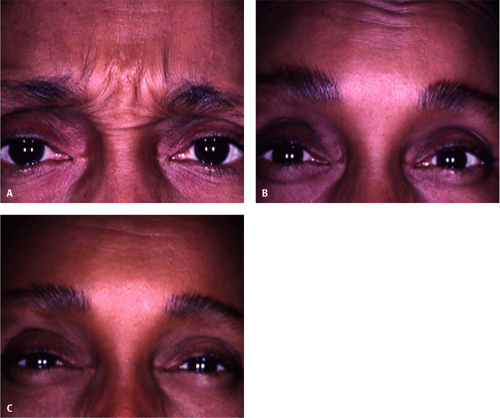
Independent of skin color, all patients develop the same response to BT injections. In the phase III BT type A clinical trials, 21 of the 405 enrolled were African American. An analysis was conducted looking at the overall efficacy and safety in the 21 African Americans compared with Caucasian subjects. There were no statistically significant differences in the efficacy or safety responses80 (Fig. 22-4A–C).