Seward JF, Galil K, Damon I, Norton SA, Rotz L, Schmid S, et al. Clin Infect Dis 2004; 39; 1477–83. Diagnostic criteria and a CDC algorithm to evaluate and manage suspected cases of smallpox. Three categories (i.e., low, moderate, or high risk of actually having smallpox) dictate subsequent diagnostic strategies. Specific variola laboratory testing is reserved for high-risk persons. An interactive version of the algorithm is available online at http://www.bt.cdc.gov/agent/smallpox/diagnosis/riskalgorithm/index.asp.
Bioterrorism
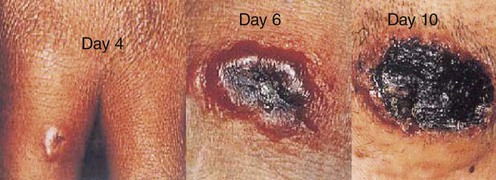
Smallpox
Specific investigations
First-line therapies
Bioterrorism




















