Basic Pathologic Reactions of the Skin: Introduction
|
The skin is composed of different tissue compartments that interconnect anatomically and interact functionally. It is difficult to envisage epidermal function without signals from the dermis or passenger leukocytes traveling to and from the skin. On the other hand, epidermis, dermis, and subcutaneous tissue are heterogeneous in nature and an analysis of pathologic processes involving the skin should therefore consider both this heterogeneity and the interactions of the individual cutaneous compartments; only then will it be understood why a few basic reactions lead to a multiplicity of reaction patterns within this tissue.
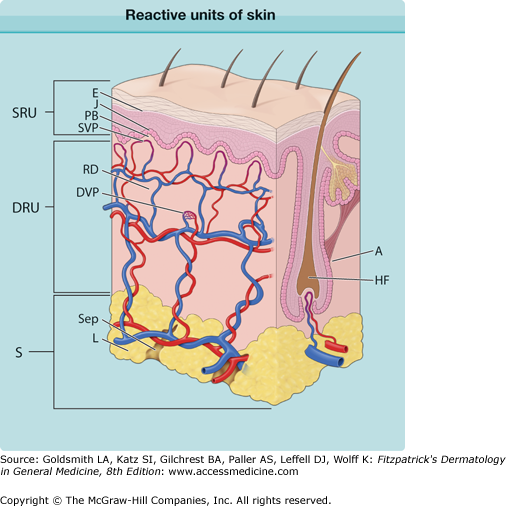
Pathophysiologically, the skin can be subdivided into three reactive units that extend beyond anatomic boundaries (Fig. 6-1); they overlap and can be divided into different subunits that respond to pathologic stimuli according to their inherent reaction capacities in a coordinated pattern.
Figure 6-1
Reactive units of skin. The superficial reactive unit (SRU) comprises the epidermis (E), the junction zone (J), and the papillary body (PB) with the superficial microvascular plexus. The dermal reactive unit (DRU) consists of the reticular dermis (RD) and the deep dermal microvascular plexus (DVP). The subcutaneous reactive unit (S) consists of lobules (L) and septae (Sep). A fourth unit is the appendages (A; hair and sebaceous glands are the only appendages shown). HF = hair follicle.
The superficial reactive unit comprises the subunits epidermis, the junction zone, the subjacent loose, delicate connective tissue of the papillary body and its capillary network, and the superficial vascular plexus (see Fig. 6-1, SRU). The reticular layer of the dermis represents a second reactive unit and is composed of coarse connective tissue and the deeper dermal vascular plexus (see Fig. 6-1, DRU). The third reactive unit, the subcutaneous tissue, is also anatomically and functionally heterogeneous; septal and lobular compartments may be involved either alone or together (see Fig. 6-1, S). Hair follicles and glands are a separate (fourth) reactive unit embedded in these three basic units.
Superficial Reactive Unit
(See Fig. 6-1, E)
Keratinocytes, which have the capacity to synthesize keratin protein, represent the bulk of the epidermis. The epidermis, an ectodermal epithelium, also harbors a number of other cell populations such as melanocytes, Langerhans cells, Merkel cells, and cellular migrants (see Chapter 7). The basal cells of the epidermis undergo proliferation cycles that provide for the renewal of the epidermis and, as they move toward the surface of the skin, undergo a differentiation process that results in surface keratinization. Thus, the epidermis is a dynamic tissue in which cells are constantly in nonsynchronized replication and differentiation; this precisely coordinated physiological balance between progressive keratinization as cells approach the epidermal surface to eventually undergo programed cell death and be sloughed, and their continuous replenishment by dividing basal cells is in contrast to the relatively static minority populations of Langerhans cells, melanocytes, and Merkel cells. However, at the same time, these dynamic keratinocytes are interconnected through cohesive molecular interactions that guarantee the continuity, stability, and integrity of the epithelium. Stability for this directional cellular flow is provided by the basal membrane complex (see Chapter 53), which anchors the epidermis to the dermis, and the stratum corneum, which encases the epidermis on the outside. It is here that individual cell differentiation ceases as the keratinizing cells are firmly interconnected by an intercellular cement-like substance forming a permeability barrier (see Chapter 47). These forces of cohesion are finally lost at the surface of the epidermis where the individual cornified cells are sloughed (desquamated). Therefore, pathologic changes within the epidermis may relate to the replicative kinetics of epidermal cells, their differentiation, alterations in cohesive forces, or a combination of these factors (see Chapter 46). These primary factors may also influence the stability and migratory characteristics of Langerhans cell, melanocytes, and migrant lymphocytes, accounting for the complexity of certain reaction patterns that arise from primary pathological defects in the epidermal layer. For example, unless a Langerhans cell expresses the chemokine receptor CCR6, it cannot migrate from the dermis to the epidermis, and without expression of the CCR7 receptor, migration to the lymph node is not possible. Because cytokines that regulate the expression of such receptors are synthesized and secreted by keratinocytes within the immediate microenvironment of Langerhans cells, impairment of keratinocyte homeostasis may have far-reaching functional implications that are reflected in the complexity of the resultant reaction patterns.
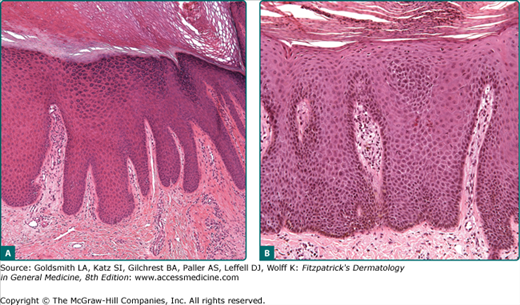
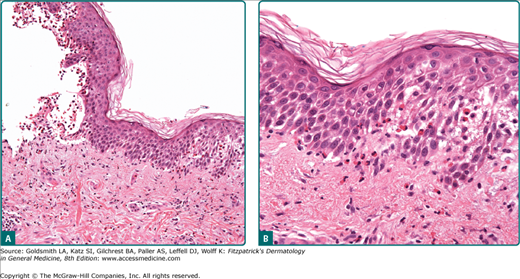
The mitotic rate of germinative basal cells, the desquamation rate of corneocytes, and the generation time of epidermal cells determine the homeostasis of the epidermis (see Chapter 46). Under physiologic conditions, there is a balance among proliferation, differentiation, and desquamation. Enhanced cell proliferation accompanied by an enlargement of the germinative cell pool and increased mitotic rates lead to an increase of the epidermal cell population and thus to a thickening of the epidermis (acanthosis) (Fig. 6-2A). A shift in the ratio of resting to proliferating cell as is the case in psoriasis (see Chapter 18) will lead to both an increase in the turnover of the entire epidermis and to a considerable increase of the volume of germinative cells that have to be accommodated at the dermal–epidermal junction. This, in turn, results in a widening and elongation of the rete ridges, which is accompanied by a compensatory elongation of the connectivetissue papillae, resulting in an expansion of the dermal–epidermal interface and, consequently, in an increased surface area for dermal–epidermal interactions (see Fig. 6-2).
In contrast to acanthosis is epidermal atrophy. Although there are many causes, one primary etiology is a decrease in epidermal proliferative capacity, as may be seen with physiological aging or after the prolonged use of potent topical or systemic steroids. With atrophy, the epidermal rete ridges are initially lost, followed by progressive thinning of the epidermal layer. Depending on the underlying causes and how they affect the keratinocyte differentiation program, there may also be hyperkeratosis or parakeratosis (thickening of the stratum corneum or retention of nuclei into the stratum corneum, respectively). It is likely that many forms of acanthosis and atrophy have primary effects of the homeostasis and function of keratinocyte stem cells, critically important slow-cycling minority populations of epidermal cells that are normally sequestered in the bulge areas of hair follicles and at tips of epidermal rete ridges.
A simple example of disturbed epidermal differentiation is parakeratosis, in which faulty and accelerated cornification leads to a retention of pyknotic nuclei of epidermal cells at the epidermal surface that is normally formed by anucleate cell remnants that form a “basket-weave” architectural pattern (see Fig. 6-2B). A parakeratotic stratum corneum is not a continuous sheet of cornified cells but a loose structure with gaps between cells; these gaps lead to a loss of the barrier function of the epidermis.
Parakeratosis can be the result of incomplete differentiation in postmitotic germinative cells due to factors that influence the timing and complex integrity of the differentiation program whereby keratin pairs of relatively low molecular weight are progressively assembled as cells approach the epidermal surface. Alternatively, parakeratosis can also be the result of reduced transit time, which does not permit epidermal cells to complete the entire differentiation process, as for example in psoriasis. However, “parakeratosis” of cellophane-stripped epidermis becomes microscopically visible as early as 1 hour after trauma; here, parakeratosis does not represent disturbed differentiation; rather, it results from direct cellular injury to a viable epidermis deprived of its protective horny layer. Therefore, the morphologic term parakeratosis may signify a programed disturbance of differentiation and maturation, alterations in cell replication kinetics, or direct cellular injury.
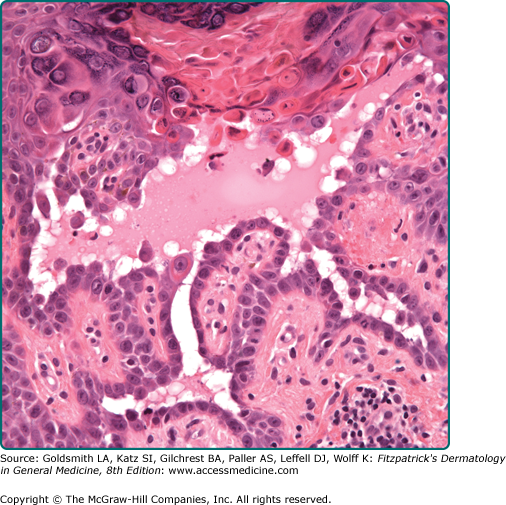
Dyskeratosis represents altered, often premature or abnormal, keratinization, of individual keratinocytes but it also refers to the morphologic presentation of apoptosis of keratinocytes. Dyskeratotic cells have an eosinophilic cytoplasm and a pyknotic nucleus and are packed with keratin filaments arranged in perinuclear aggregates. Such a cell will tend to round up and lose its attachments to the surrounding cells. Therefore, dyskeratosis is often associated with acantholysis (see Section “Disturbances of Epidermal Cohesion”) but not vice versa (Fig. 6-3).
In some diseases, dyskeratosis is the expression of a genetically programed disturbance of keratinization as is the case in Darier disease (see Chapter 51). Dyskeratosis may occur in actinic keratosis and squamous cell carcinoma. Dyskeratosis may also be caused by direct physical and chemical injuries. In the sunburn reaction, eosinophilic, apoptotic cells—so-called sunburn cells—are found within the epidermis within the first 24 hours after irradiation with ultraviolet B (UVB) (see Chapter 90), and similar cells may occur after high-dose systemic cytotoxic treatment. Individual cell death within the epidermis is a regular phenomenon in graft versus host reactions of the skin (see Chapter 28) and in erythema multiforme (see Chapter 39). It is important to remember that although both premature or abnormal keratinization and apoptosis may produce an end product referred to as “dyskeratosis,” the early events and mechanisms responsible are different. Whereas cells early in the process of abnormal keratinization often have increased eosinophilic keratin aggregates within their cytoplasm with viable-appearing nuclei, apoptotic cells during early evolutionary stages have shrunken, pyknotic, and sometimes fragmented nuclei in the setting of normal-appearing cytoplasm.
Epidermal cohesion is the result of a dynamic equilibrium of forming and dissociating intercellular contacts. Specific intercellular attachment devices (desmosomes) and the related intercellular molecular interactions are responsible for intercellular cohesion. However, epidermal cohesion must permit epidermal cell motion. Desmosomes dissociate and reform at new sites of intercellular contact as cells migrate through the epidermis and keratinocytes mature toward the epidermal surface. Intercellular cohesive forces are strong enough to guarantee the continuity of the epidermis as an uninterrupted epithelium but, on the other hand, are adaptable enough to permit locomotion, permeability of the intercellular space, and intercellular interactions.
The most common result of disturbed epidermal cohesion is the intraepidermal vesicle, a small cavity filled with fluid. A classification of intraepidermal blistering by anatomic level is shown in Table 6-1.
Granular layer |
Friction blister |
Pemphigus foliaceus |
Subcorneal pustular dermatosis |
Staphylococcal scalded-skin syndrome/bullous impetigo |
Spinous layer |
Eczematous dermatitis |
Herpes virus infection |
Familial benign pemphigus |
Suprabasal |
Pemphigus vulgaris |
Darier disease |
Basal layer |
Erythema multiforme |
Lupus erythematosus |
Lichen planus |
Epidermolysis bullosa |
Three basic morphologic patterns of intraepidermal vesicle formation are classically recognized. Spongiosis is an example of the secondary loss of cohesion between epidermal cells due to the influx of tissue fluid into the epidermis. Serous exudate may extend from the dermis into the intercellular compartment of the epidermis; as it expands, epidermal cells remain in contact with each other only at the sites of desmosomes, acquiring a stellate appearance and giving the epidermis a sponge-like morphology (spongiosis). As the intercellular edema increases, individual cells rupture and lyse, and microcavities (spongiotic vesicles) result (Fig. 6-4). Confluence of such microcavities leads to larger blisters. Epidermal cells may also be separated by leukocytes that disturb intraepidermal coherence; thus, the migration of leukocytes into the epidermis and spongiotic edema are often a combined phenomenon, best illustrated by acute allergic contact dermatitis. The accumulation of polymorphonuclear leukocytes within the epidermis, the resulting separation of epidermal cells, and their subsequent destruction by neutrophil-derived enzymes, eventually lead to the formation of a spongiform pustule, one of the histopathologic hallmarks of psoriasis (see Chapter 18).
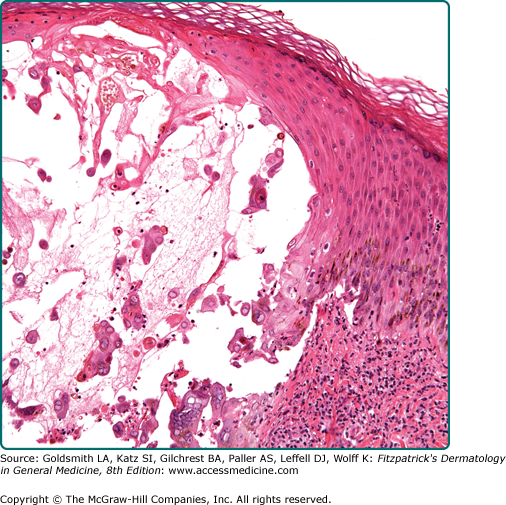
Acantholysis is a primary loss of cohesion of epidermal cells. This is initially characterized by a widening and separation of the interdesmosomal regions of the cell membranes of keratinocytes, followed by splitting and a disappearance of desmosomes (see Chapter 53). The cells are intact but are no longer attached; they revert to their smallest possible surface and round up (Figs. 6-3 and 6-5). Intercellular gaps and slits result, and the influx of fluid from the dermis leads to a cavity, which may form in a suprabasal (Fig. 6-6), midepidermal, or even subcorneal location. Acantholytic cells can easily be demonstrated in cytologic smears (see Fig. 6-5) and in some conditions have diagnostic significance.
Acantholysis occurs in a number of different pathologic processes that do not have a common etiology and pathogenesis. Acantholysis may be a primary event leading to intraepidermal cavitation (primary acantholysis) or a secondary phenomenon in which epidermal cells are shed from the walls of established intraepidermal blisters (secondary acantholysis). Primary acantholysis is a pathogenetically relevant event in diseases of the pemphigus group (see Chapter 54), in which it results from the interaction of autoantibodies and antigenic determinants on the keratinocyte membranes and related desmosomal adhesive proteins, and in the staphylococcal scalded-skin syndrome, where it is caused by a staphylococcal exotoxin (epidermolysin) (see Chapter 177). In familial benign pemphigus, it results from the combination of a genetically determined defect of the keratinocyte cell membrane and exogenous factors (see Chapter 51). A similar phenomenon, albeit more confined to the suprabasal epidermis, occurs in Darier disease, where it is combined with dyskeratosis in the upper epidermal layers (see Fig. 6-3) and a compensatory proliferation of basal cells into the papillary body (see Chapter 51). When acantholysis results from viral infection, it is usually combined with other cellular phenomena such as ballooning, giant cells, and cytolysis (Fig. 6-7; see Chapters 193 and 194).
Indeed, a loss of epidermal cohesion can also result from a primary dissolution of cells (i.e., cytolysis). In the epidermolytic forms of epidermolysis bullosa, genetically defective and thus structurally compromised basal cells rupture as a result of trauma so that the cleft forms through the basal cell layer independently from preexisting anatomic boundaries (see Chapter 62). Cytolytic phenomena in the stratum granulosum are characteristic for epidermolytic hyperkeratosis, bullous congenital ichthyosiform erythroderma, ichthyosis hystrix, and some forms of hereditary palmoplantar keratoderma (see Chapters 49 and 50).
(See Fig. 6-1, J)
Epidermis and dermis are structurally interlocked by means of the epidermal rete ridges and the corresponding dermal papillae, and foot-like submicroscopic cytoplasmic microprocesses of basal cells that extend into corresponding indentations of the dermis. Dermal–epidermal attachment is enforced by hemidesmosomes that anchor basal cells onto the basal lamina; this, in turn, is attached to the dermis by means of anchoring filaments and microfibrils (see Chapter 53). These structural relationships correlate with complex molecular interactions that serve to bind the epidermis, basement membrane, and superficial dermis in a manner that promotes resistance to potentially life-threatening epidermal detachment. The basal lamina is not a rigid or impermeable structure because leukocytes, Langerhans cells, or other migratory cells pass through it without causing a permanent breach in the junction. After being destroyed by pathologic processes, the basal lamina is reconstituted; this represents an important phenomenon in wound healing and other reparative processes. Functionally, the basal lamina is part of a unit that, by light microscopy, appears as the periodic acid-Schiff–positive “basement membrane” and, in fact, represents the entire junction zone. This consists of the lamina lucida, spanned by microfilaments, and subjacent anchoring fibrils, small collagen fibers, and extracellular matrix (see Chapter 53). The junction zone is a functional complex that is primarily affected in a number of pathologic processes.
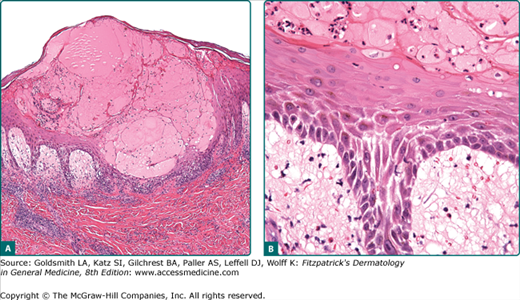
The destruction of the junction zone or its components usually manifests as disturbance of dermal–epidermal cohesion and leads to blister formation. These blisters appear to be subepidermal by light microscopy (Fig. 6-8), but in reality may be localized at different levels and result from pathogenetically heterogeneous processes. A classification of blisters at the junction by anatomic level is given in Table 6-2. Subepidermal blister formation occurs in forms of epidermolysis bullosa (see Chapter 62) or can be the result of a complex inflammatory process that involves the entire junction zone, as is the case in lupus erythematosus, erythema multiforme, or lichen planus; therefore, it may be a phenomenon occurring in a group of etiologically and pathogenetically heterogeneous conditions. In bullous pemphigoid (see Fig. 6-8), cleft formation runs through the lamina lucida of the basal membrane and is caused by autoantibodies directed against specific antigens on the cytomembrane of basal cells (junctional blistering) (see Fig. 6-8A; see Chapter 56). The presence of eosinophil granules that contain major basic protein that is toxic to keratinocytes also causes keratinocyte injury and may present as eosinophilic apongiosis (Fig. 6-8B). Junctional blistering also occurs in the junctional forms of epidermolysis bullosa, but here it is due to the hereditary impairment or absence of molecules important for dermal–epidermal cohesion (see Chapter 62; see Table 6-2).
Junctional (at the lamina lucida) |
Junctional epidermolysis bullosa |
Bullous pemphigoid |
Dermolytic (below basal lamina) |
Epidermolysis bullosa dystrophicans |
Epidermolysis bullosa acquisita |
Porphyria cutanea tarda |