Figure 21-1 Impetigo contagiosa. A: In this relatively early lesion, neutrophils are seen in the upper epidermis, forming a subclone pustule. There is a superficial mixed infiltrate within the dermis. B: On closer inspection, neutrophils and bacterial colonies are seen within the stratum corneum. C: Yellow crusted vesiculopustules on the back of the hand of a child. (Part C image courtesy of C. Kovarik.)
The stratum malpighii underlying the bulla is spongiotic, and neutrophils often can be seen migrating through it. The upper dermis contains a moderately severe inflammatory infiltrate of neutrophils and lymphoid cells.
At a later stage, when the vesicle has ruptured, the horny layer is absent, and a crust composed of serous exudate and the nuclear debris of neutrophils may be seen covering the stratum malpighii.
Pathogenesis. In the United States, group A streptococci used to be the most frequently recovered organisms in patients with impetigo contagiosa, either alone or in association with S. aureus. However, the predominant pathogen responsible for impetigo has changed, and S. aureus is the most common organism in the United States and United Kingdom (6). Cultures of such lesions are vital in order to identify methicillin-resistant S. aureus (MRSA) as the causal agent versus other antibiotic-susceptible skin and environmental flora.
Differential Diagnosis. Histologic differentiation of impetigo contagiosa from subcorneal pustular dermatosis, pemphigus foliaceus, and IgA pemphigus can be difficult. However, only impetigo shows gram-positive cocci in the bulla cavity on routine Gram staining. In addition, pemphigus foliaceus usually shows fewer neutrophils, more acantholytic cells, and occasionally some dyskeratotic granular cells.
Bullous Impetigo and Staphylococcal Scalded-Skin Syndrome
Clinical Summary. Phage group II staphylococci may produce bullous impetigo and the staphylococcal scalded-skin syndrome.
Bullous impetigo occurs mainly in newborns, infants, and young children. Occasionally, it is observed in adults, particularly in those with deficiencies in cell-mediated immunity (7,8). It is characterized by vesicles that rapidly progress to flaccid bullae with little or no surrounding erythema. The contents of the bullae are initially clear but may progress to turbid. There is often a distinctive honey-colored dried serous crust over inflamed skin. Bullous impetigo may spread and become generalized; so clinical distinction from staphylococcal scalded-skin syndrome may be impossible (9). An important difference, however, is that cultures from intact bullae of bullous impetigo, unlike those of the staphylococcal scalded-skin syndrome, grow phage group II S. aureus.
Staphylococcal scalded-skin syndrome, first described more than 100 years ago and also known as Ritter disease, occurs largely in the newborn and in children younger than 5 years. It rarely occurs in adults or in older children (10–12) except in the presence of severe underlying disease such as renal insufficiency, which leads to decreased toxin clearance (13).
The disease begins abruptly with diffuse erythema and fever. Large, flaccid bullae filled with clear fluid form and rupture almost immediately. Large sheets of superficial epidermis separate and exfoliate. The disease runs an acute course and is fatal in more than 4% of all cases in children (14). Most fatalities occur in neonates with generalized lesions. In contrast, in the rare cases of staphylococcal scalded-skin syndrome occurring in adults, the prognosis is much worse, with a mortality rate exceeding 50%. Death is usually related to the coexistent disease or to immunosuppressive therapy given for it. Both bullous impetigo and staphylococcal scalded-skin syndrome are transmissible and can cause epidemics in nurseries, where they may occur together (15).
The absence of phage group II staphylococci from the bullae of staphylococcal scalded-skin syndrome results from the fact that these staphylococci are present at a distant focus. Usually, the distant focus is extracutaneous and consists of a purulent conjunctivitis, rhinitis, or pharyngitis. Rarely, the distant focus consists of a cutaneous infection or a septicemia.
Histopathology. In both bullous impetigo and staphylococcal scalded-skin syndrome, the cleavage plane of the bulla, like that in impetigo contagiosa, lies in the uppermost epidermis either below or, less commonly, within the granular layer (Fig. 21-2). A few acantholytic cells are often seen adjoining the cleavage plane. However, in contrast to impetigo contagiosa, there are few or no inflammatory cells within the bulla cavity. In bullous impetigo, the upper dermis may show a polymorphous infiltrate, whereas in the staphylococcal scalded-skin syndrome, the dermis is usually free of inflammation.
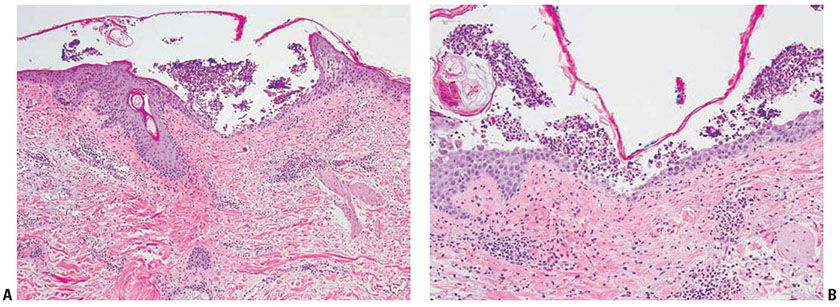
Figure 21-2 Bullous impetigo. A: Subcorneal pustular infiltrate is characteristic of bullous impetigo. B: Notice the prominent neutrophilic infiltrate and relative absence of dyskeratotic cells, which is helpful in distinguishing it from pemphigus follicaceous.
Pathogenesis. The causative epidermolysins are the exfoliative toxins (ETs) produced by S. aureus. There are many strains of these serine protease enzymes. Their target is desmoglein 1, a desmosomal glycoprotein, which has a role in cell-to-cell adhesion in the superficial epidermis (16).
Electron microscopy shows that the cleavage plane of lesions in humans as well as in mice is at the interface between the spinous and granular layers, with some upward extension into the lower granular layer. Splitting occurs without damage to adjacent acantholytic keratinocytes. ET appears to act primarily on the intercellular substance, because in studies carried out on newborn mice, the intercellular spaces widen and microvilli form before the desmosomes separate within their interdesmosomal contact zone (17).
Differential Diagnosis. Staphylococcal scalded-skin syndrome and toxic epidermal necrolysis of Lyell type show clinically extensive detachment of the epidermis and thus may clinically resemble one another. This has resulted in confusion in the past; at one time, both diseases were referred to as Lyell disease or toxic epidermal necrolysis. As the target of staphylococcal toxin is desmoglein 1, clinically the epidermal split in this condition is superficial, leaving pink intact epidermis under superficially peeling skin, as opposed to toxic epidermal necrolysis, wherein the entire epidermis is diseased and detached, leaving moist, inflamed, raw dermis visible under the denuded areas. Similarly, staphylococcal scalded-skin syndrome should not affect the mucosa, while Stevens–Johnson syndrome and toxic epidermal necrolysis almost always do. Additionally, the two diseases can be easily differentiated histologically; in severe erythema multiforme/Stevens–Johnson syndrome, the entire, or nearly the entire, epidermis detaches itself, with considerable necrosis of the epidermal cells, whereas in staphylococcal scalded-skin syndrome, only the uppermost portion of the epidermis becomes detached, with relatively slight damage to the underlying epidermal cells. This difference can be readily identified with frozen section analysis of denuded detached skin for rapid diagnosis.
Principles of Management. The major objectives of impetigo treatment (including bullous impetigo) are reducing the spread of infection and supporting the resolution of discomfort and cosmetic appearance. Oral antimicrobial therapy with activity against β-hemolytic streptococci and S. aureus for 7 days is usually sufficient. Topical therapy, such as mupirocin ointment, may be administered in cases with a limited number of lesions without bullae. Hand washing is important for reducing the spread in children (18). Staphylococcal toxic shock syndrome (TSS) requires aggressive multidisciplinary care with supportive care and often parenteral antibiotics.
Methicillin-Resistant S. Aureus
Clinical Summary. Originally described shortly after the introduction of methicillin in 1961, the prevalence of MRSA has increased in both health care and community settings. MRSA skin infections are currently classified as health care-associated (HA-MRSA) or community-associated (CA-MRSA), based on the presence or absence of health care exposure. Evolving epidemiologic observations suggest that there is no clear distinction between these two categories, since patients can develop MRSA colonization in one realm and develop signs and symptoms of infection in the other. HA-MRSA infections have been observed with increasing frequency in patients in community settings, and similarly, infection by “community-associated” strains have become more common among patients in hospital settings. Moreover, studies suggest that CA-MRSA strains may be replacing traditional hospital-acquired strains. Nevertheless, there are important clinical and molecular epidemiologic differences that merit consideration (19). Infection caused by CA-MRSA is considered to represent a worldwide epidemic, often presenting as a skin and soft tissue infection in young, otherwise healthy individuals. Athletes, certain ethnic populations, children, homeless persons, homosexual men, household members of infected people, HIV-infected patients, intravenous drug abusers, military personnel, newborns, pregnant and postpartum women, tattoo recipients, and urban dwellers of lower socioeconomic status in crowded living conditions are said to be at increased risk of developing CA-MRSA infection. A recent study conducted in primary care clinics in Texas found CA-MRSA to be the predominant pathogen implicated in skin and soft tissue infections in the ambulatory setting (20). Colonization by S. aureus in patients whose skin barrier is disrupted represents a potential reservoir for CA-MRSA. In addition, infection-associated risk factors are absent in many individuals who develop cutaneous CA-MRSA infection. The most common presentations of CA-MRSA infection are abscess, cellulitis, or both, not uncommonly misinterpreted by the patient as insect bites. Other manifestations of cutaneous CA-MRSA infection are impetigo, folliculitis, and paronychia.
Hospital-associated MRSA is defined as MRSA infection that occurs 48 hours or later following hospitalization or infection occurring outside of the hospital within 12 months of exposure to health care (e.g., surgery). It is often more virulent than CA-MRSA. Patients may be at risk of developing not just skin and soft tissue infections but bacteremia, sepsis, and seeding of internal devices, hardware, heart valves, and joints. Risk factors for HA-MRSA infection include prolonged hospitalization, antibiotic use, admission to intensive care, and hemodialysis among others. Many hospitals routinely screen all patients admitted to identify carriers, and enact strict contact isolation to limit the spread of MRSA through the hospital. This form of MRSA may display substantially different antibiotic resistance patterns, and cultures to identify antibiotic sensitivities are essential to treatment.
Histopathology. The histologic findings are nonspecific, and correlation with microbiologic cultures is essential to establish a diagnosis of certitude. Prominent dermal edema and lymphatic dilatation may be observed. A diffuse inflammatory infiltrate around blood vessels, predominantly of polymorphonuclear neutrophils, may be present. Granulation tissue with a mononuclear infiltrate of lymphocytes and histiocytes is seen in later stages. A Gram stain may reveal abundant cocci in florid infections.
Pathogenesis. Methicillin resistance is encoded by the mecA gene on the staphyloccocal chromosome cassette (SCCmec), encoding PBP-2a, a penicillin-binding protein that allows the microorganism to grow and divide in the presence of methicillin or other β-lactam antibiotics. By 2004, six major clones of MRSA had emerged worldwide, labeled SCCmecI–VI. Dissemination of resistance is mediated by horizontal transfer of the mecA gene (19).
Principles of Management. Incision and drainage of abscesses, systemic antibacterial therapy, and adjunctive topical antibacterial treatment are the essential components of management of CA-MRSA skin infections. The spread of cutaneous CA-MRSA infection can potentially be prevented by a combination of personal, environmental, and health care measures directed at eliminating the causes of acquisition and transmission of the bacteria (19). Patients may be chronic carriers, and staph eradication may be necessary with antimicrobial bathing, intranasal, perineal, and subungual topical antistaph antibiotics, and sometimes courses of combination oral antibiotics to reduce the carrier state. It is important for clinicians to be aware of MRSA resistance patterns in their community, to choose appropriate empiric antibiotics, and to tailor therapy based on culture data. For HA-MRSA, patients often require IV antibiotics and may need an infectious disease specialist to assist in selecting medications and determining therapeutic duration.
BLASTOMYCOSIS-LIKE PYODERMA (PYODERMA VEGETANS)
Clinical Summary. Two entirely different diseases have been described under the term pyoderma vegetans or pyodermite végétante of Hallopeau. One disease, now referred to as pemphigus vegetans of Hallopeau, shows the typical intercellular immunofluorescence of the pemphigus group on direct immunofluorescence testing. The other disease represents a vegetating tissue reaction, possibly secondary to bacterial infection (21). In order to emphasize the difference between it and pemphigus vegetans of Hallopeau, it is preferable to refer to this disease as blastomycosis-like pyoderma rather than as pyoderma vegetans (22).
Blastomycosis-like pyoderma shows one or multiple large, verrucous, vegetating plaques with scattered pustules and elevated borders. The plaques show considerable resemblance to those observed in fungal blastomycosis. The location of the plaques varies considerably from case to case. In some instances, the face and legs are affected; in others it is the intertriginous areas. Some authors have observed an association with ulcerative colitis (23).
Histopathology. The two major features of blastomycosis-like pyoderma are pseudocarcinomatous hyperplasia and multiple abscesses in the dermis as well as in the hyperplastic epidermis. The abscesses are composed of neutrophils in some cases and of eosinophils in others.
Pathogenesis. Bacteria, most commonly S. aureus, can be found regularly in blastomycosis-like pyoderma; however, the presence of several different strains and the variable response of patients to antibiotic therapy suggest that the bacteria are secondary invaders, although they may be responsible for the vegetating tissue reaction. A deficiency in cellular immunity (24) and a decrease in the chemotactic activity of the neutrophils have also been observed.
Differential Diagnosis. In cases with largely eosinophils in the abscesses, pemphigus vegetans must be excluded by direct immunofluorescence. If there is marked pseudocarcinomatous hyperplasia, multiple biopsies may be necessary for differentiation from true squamous cell carcinoma.
Principles of Management. There is no standardized treatment plan available for pyoderma vegetans. Antimicrobial therapy has been used, with variable results. Alternatives include curettage and topical application of aluminum subacetate soaks. Attention to underlying systemic illness should also be considered (21).
ERYSIPELAS
Clinical Summary. Erysipelas, once termed “St. Anthony’s fire,” is an acute superficial cellulitis of the skin caused by β-hemolytic streptococci. It is characterized by the presence of a well-demarcated, slightly indurated, dusky red patch or thin plaque with an advancing, palpable border. In some patients, erysipelas has a tendency to recur periodically in the same areas. Recent data suggest a significant association between several single nucleotide polymorphisms (SNPs) in the promoter of the AGTR1 gene (angiotensin II receptor type I) and susceptibility to erysipelas in humans (25). In the early antibiotic era, the incidence of erysipelas appeared to be on the decline, and most cases occurred on the face. More recently, however, there appears to have been an increase in the incidence, and facial sites are now less common, whereas erysipelas of the legs is predominant. Potential complications in patients with poor resistance or after inadequate therapy may include abscess formation, spreading necrosis of the soft tissue, infrequently necrotizing fasciitis, and septicemia (26). Nephritic and cardiac complications are rare, because erysipelas is usually produced by nonnephritogenic and nonrheumatogenic strains of streptococci.
Histopathology. The findings are nonspecific, and the diagnosis relies on clinical and microbiologic culture correlation. The dermis shows marked edema and dilatation of the lymphatics and capillaries. There is a diffuse infiltrate, composed chiefly of neutrophils, that extends throughout the dermis and occasionally into the subcutaneous fat. It shows a loose arrangement around dilated blood and lymph vessels. If sections are stained with the Giemsa or Gram stain, streptococci are found in the tissue and within lymphatics. In cases of recurring erysipelas, the lymph vessels of the dermis and subcutaneous tissue show fibrotic thickening of their walls with partial or complete occlusion of the lumen.
Pathogenesis. Erysipelas and cellulitis are infections that develop as a result of bacterial entry via breaches in the skin barrier. The vast majority of cases are caused by β-hemolytic streptococci (groups A, B, C, G, and F) and S. aureus, including MRSA. Gram-negative aerobic bacilli are isolated in a minority of cases. Less common pathogens include Haemophilus influenzae (buccal cellulitis), clostridia and non–spore-forming anaerobes (crepitant cellulitis), pneumococcus and meningococcus.
Principles of Management. Patients with classic manifestations of erysipelas are best treated with parenteral antimicrobial therapy, with ceftriaxone or cephazolin being choices with appropriate coverage. Patients with mild infection or after improvement with parenteral therapy may be treated with oral β-lactam antimicrobials. Supportive measures such as elevation of affected area and treatment of underlying predisposing conditions should be included. Exacerbation of erythema after initiation of antimicrobial therapy may be observed as a result of pathogen destruction and enzymatic release, and should not be mistaken for failure to respond (26).
TOXIC SHOCK SYNDROME
TSS is an acute febrile illness usually caused by group A β-hemolytic Streptococcus and certain toxin-producing strains of S. aureus (27).
Clinical Summary. In the initial report in 1978, the disease was described in a series of seven children (28). Although many subsequent cases have been associated with the use of superabsorbent vaginal tampons by menstruating women, additional reported settings for TSS have included surgical wound infections of the skin (29), empyema, fasciitis, osteomyelitis, peritonsillar abscesses, and other infections (30). Fever, hypotension or shock, an extensive rash resembling scarlet fever or sunburn, and involvement of three or more organ systems are the initial defining manifestations, whereas desquamation occurs 1 to 2 weeks after the onset. In rare instances, bullae are also present (31). In addition, there may be a conjunctivitis and an oropharyngitis. Internal organs may be affected by toxic encephalopathy, thrombocytopenia, renal failure, or hepatic damage. Mortality has been estimated at 5% to 10% (32).
Histopathology. The histologic findings are nonspecific. A superficial perivascular and interstitial mixed-cell infiltrate containing neutrophils and sometimes eosinophils, foci of spongiosis containing neutrophils, and scattered necrotic keratinocytes that sometimes are arranged in clusters within the epidermis has been described (33). If bullae are present, they are subepidermal in location.
Pathogenesis. Even though in rare cases S. aureus bacteremia occurs, the cause of staphylococcal TSS is a toxin that is produced locally at the site of a toxigenic infection. Several toxins have been implicated in TSS, including toxic shock syndrome toxin 1 (TSST-1) and the staphylococcal enterotoxins B, C, and F (34). Susceptibility to disease correlates with absence of protective antibodies to the toxin, and cytokine activation may be involved in some aspects of its pathogenesis. If a pure culture of S. aureus is obtained from a suspected patient, the bacteria can be tested for the presence of the toxin to confirm diagnosis. Streptococcal TSS has been associated with bacteremia and extensive necrotizing fasciitis, unlike the staphyloccocal syndrome usually associated with occult or minor focal infections. Streptococcal TSS appears to be caused also by a variety of toxins produced by the bacteria including pyrogenic exotoxin A and/or B, streptococcal superantigen, and mitogenic factor (35). As with S. aureus, isolates of streptococci from suspected patients may be tested for the presence of specific toxins (36).
Differential Diagnosis. A petechial rash mimicking meningococcemia is a rare manifestation of streptococcal TSS. Bacteremia is uncommon in staphylococcal TSS, in contrast to streptococcal TSS. In patients with fulminant presentations and suspicion for a possible skin infection, consideration should be given as to whether the patient may have necrotizing fasciitis, which represents both a medical and surgical emergency and requires immediate intervention.
Principles of Management. The mainstay of therapy for staphylococcal TSS is supportive with fluid replacement, which may be extensive at times to maintain perfusion in the setting of hypotension. Vasopressors may also be required. Foreign material should be sought after and removed if present (i.e., tampons, contraceptive sponges). While it is not clear whether antibiotics alter the course of acute TSS, most patients receive broad IV antibiotics during their acute illness. Notably, antistaphylococcal antibiotic therapy is required to eradicate organisms and prevent recurrences (37). Streptococcal TSS requires aggressive, combined antibiotics, with patients often receiving third-generation cephalosporins, clindamycin (which may impair bacterial ribosomal protein production and theoretically reduce toxin formation), and vancomycin to cover for the possibility of resistant staphylococcal infections. Intravenous Immunoglobulin (IVIg) is controversial and has been variably used in an effort to decrease the inflammatory response.
MYCOBACTERIAL INFECTIONS
Taxonomically, the mycobacteria are divided into two major groups: the rapid growers and the slow growers in culture. Mycobacterium leprae lies outside this scheme because it cannot be cultured in vitro. Within each group, the species are organized in subgroups according to certain culture (growth requirement) and biochemical characteristics. Among the infections to be considered in this chapter, the important slow growers are the M. tuberculosis complex, including Bacille Calmette–Guérin (BCG); the M. avium complex, including M. avium and M. avium-intracellulare; the M. kansasii complex; M. marinum; M. ulcerans; and mycobacteria with special growth requirements, such as M. haemophilum. Rapid-growing mycobacteria (RGM) include three clinically relevant species: M. fortuitum, M. chelonae, and M. abscessus (38). Many other mycobacteria can occasionally cause skin lesions (39–41). In a series of 25 cases from Israel, there were 16 cases with M. marinum, 3 of atypical Mycobacterium without species identification, and one each with M. chelonae, M. xenopi, M. abscessus, M. gordonae, and M. fortuitum, and it was emphasized that absence of a pathognomonic clinical picture and variable histologic findings often delay diagnosis of these nontuberculous mycobacteria (NTM)-induced cutaneous infections. The diagnosis of NTM should be confirmed by histology and bacteriology studies of tissue cultures. However, strong clinical suggestion of M. marinum infection may warrant initial empirical treatment to prevent progression to deep infection (42).
Tuberculosis and leprosy bacilli are intracellular parasites that are found in the tissues of humans and occasionally animals. Transmission requires exposure to infected people. The other mycobacteria are abundant in nature in both soil and water, and exposure to infection occurs throughout life, although usually without significant clinical disease.
Mycobacteria are bacilli that are weakly gram positive and are classically identified in histologic sections by stains that exploit their resistance to decoloration by acid—that is, acid-fast bacilli (43). All mycobacteria apart from the leprosy bacillus are well stained by the standard Ziehl–Neelsen technique.
LEPROSY
Clinical Summary. Leprosy, also known as Hansen disease, is a chronic granulomatous infection caused by M. leprae and that predominantly affects the skin and peripheral nerves. The disease is endemic in many tropical and subtropical countries but is declining in prevalence as a result of multidrug therapy. The Indian subcontinent, Southeast Asia, sub-Saharan countries in Africa, and Brazil comprise the areas most affected at present (44,45). The mode of transmission of leprosy is unknown, but the transmissibility is thought to be low. The mechanism is probably inhalation of bacilli, which may be excreted from the nasal passages of a multibacillary patient or possibly implanted from organisms in the soil. High numbers of bacilli of M. leprae have been documented in the nasal mucosa of infected patients (100 million bacilli) and bacterial load in the nose has been proposed to correlate with immune response in leprosy patients (46). Direct person-to-person infection by means of the skin occurs rarely if at all. After inhalation, it is likely that bacilli pass through the blood to peripheral and cutaneous nerves, where infection and host reaction commence.
Immunopathologic Spectrum of Leprosy
The sequence of disease pathogenesis is complex, is very chronic, and depends on host-mycobacteria immunologic interactions. The leprosy bacillus is nontoxic, and clinicopathologic manifestations are the result of immunopathology and/or the progressive accumulation of infected cells. Leprosy is the best example of a disease showing an immunopathologic spectrum whereby the host immune reaction to the infective agent ranges from apparently none to marked, with a consequent range of clinicopathologic manifestations (47,48). Tuberculoid leprosy indicates a high cellular immune response (i.e., T cells and macrophage activation) and few bacilli in tissues; at the opposite pole, lepromatous leprosy indicates an absent cellular immune response to M. leprae antigens, with no macrophage activation and abundant bacilli in tissues. The spectrum of leprosy is a continuum, and patients may move in either direction according to host response and treatment. The standard delineation follows that of Ridley and Jopling, with categories defined along the spectrum by a combination of clinical, microbiologic, histopathologic, and immunologic indices: TT (tuberculoid), BT (borderline tuberculoid), BB (midborderline), BL (borderline lepromatous), and LL (lepromatous). The term borderline is used to denote patterns that share some features of both tuberculoid and lepromatous leprosy (45,47).
TT and LL patients are stable, the former often self-healing and the latter remaining heavily infected unless given appropriate chemotherapy. Patients presenting at the BT point will often downgrade toward BL leprosy in the absence of treatment. The central point of the spectrum (BB) is the most unstable, with most patients downgrading to LL if not treated. The term indeterminate leprosy is used to describe patients presenting with very early leprosy lesions that cannot be categorized definitely along the immunopathologic spectrum and have the potential to self-heal or evolve into TT, BL, or LL leprosy.
It is likely that in endemic zones, a high proportion of people are infected by M. leprae but either have full immunity and no disease or have developed one or a few lesions that have self-healed without significant morbidity. The progression of infection and disease is summarized in Fig. 21-3. Patients with determined leprosy are most numerous at the BT and LL points of the spectrum.
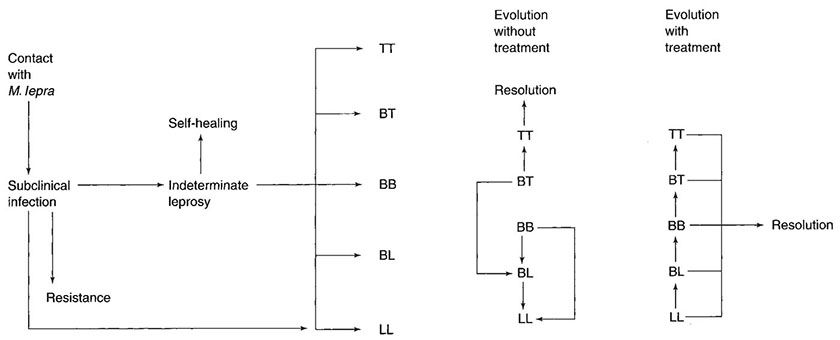
Figure 21-3 Leprosy. The sequence of events that may follow infection with M. leprae. (TT, tuberculoid; BT, borderline tuberculoid; BB, midborderline; BL, borderline lepromatous; LL, lepromatous.)
Staining of Mycobacterium Leprae Bacilli
The classical method for demonstrating leprosy bacilli in lesions is a modified Ziehl–Neelsen stain, where the degree of acid and alcohol removal of carbol fuchsin is less than in the methods used for identifying other mycobacteria. The Fite methods are the most commonly used (48). Methenamine silver stains are also useful in detecting fragmented acid-fast bacilli. The sensitivity of detection of acid-fast bacilli by histologic means remains poor, because about 1,000 bacilli per cubic centimeter of tissue must be present in order to detect one bacillus in a section. For lesions where bacilli are scanty, it is recommended that at least six sections be examined before declaring them negative (49). The standard enumeration of leprosy bacilli in lesions—the bacterial index (BI)—follows Ridley’s logarithmic scale (which applies to both skin biopsies and slit skin smears).
• BI = 0: no bacilli observed
• BI = 1: 1 to 10 bacilli in 10 to 100 high-power fields (hpf, oil immersion)
• BI = 2: 1 to 10 bacilli in 1 to 10 hpf
• BI = 3: 1 to 10 bacilli per hpf
• BI = 4: 10 to 100 bacilli per hpf
• BI = 5: 100 to 1,000 bacilli per hpf
• BI = 6: >1,000 bacilli per hpf
Solid-staining bacilli indicate that the organisms are capable of multiplication. Fragmented (beaded) and granular acid-fast bacilli indicate that they are dead. In 1998, the WHO’s Expert Committee on Leprosy determined that therapy could be initiated before smear tests or skin biopsy was performed. Consequently, a rapid means of classification for worldwide application was established based on the number of skin lesions. Patients with five or less skin lesions are termed paucibacillary, whereas patients with six or more skin lesions are termed multibacillary (45,50,51). This system has been criticized for misclassifying multibacillary patients as paucibacillary, with negative repercussions given that treatment for the paucibacillary form is only 6 months—compared to 12 for multibacillary (45,51).
Immunocytochemical methods for demonstrating mycobacterial antigens have a limited role. The most frequently used is a polyclonal anti-BCG antibody (52). In untreated lesions, it will not detect small numbers of bacilli if ordinary histochemical methods have proved negative. However, immunocytochemistry does have a role in demonstrating the presence of leprosy antigen after the bacilli have fragmented, been partly digested by macrophage enzymes, and lost their acid-fast staining quality.
Clinical Pathology of Leprosy
For general discussions of clinical leprosy and leprosy pathology, the reader is referred to Job (53) and Britton and Lockwood (45).
Early, Indeterminate Leprosy
Many patients present with obvious or advanced skin and peripheral nerve lesions (the latter are primarily nerve enlargement and the consequences of anesthesia). These patients have “determined leprosy.” However, the earliest detectable skin lesion comprises one or a few hypopigmented macules with variable loss of sensation. Any part of the body may be affected.
Histopathology. There is mild lymphocytic and macrophage accumulation around neurovascular bundles, the superficial and deep dermal vessels, sweat glands, and erector pili muscle; focal lymphocytic invasion into the lower epidermis and into the dermal nerves may be observed. No formed epithelioid cell granulomas are present (if they were, it would not be indeterminate leprosy but a TT leprosy). Schwann cell hyperplasia is a feature, but it is highly subjective. Not all of these features are present in every case. The diagnosis hinges on finding one or more acid-fast bacilli in the sites of predilection: in nerve, in erector pili muscle, just under the epidermis, or in a macrophage about a vessel. Without demonstrating bacilli, the diagnosis can only be presumptive (Figs. 21-4 and 21-5).
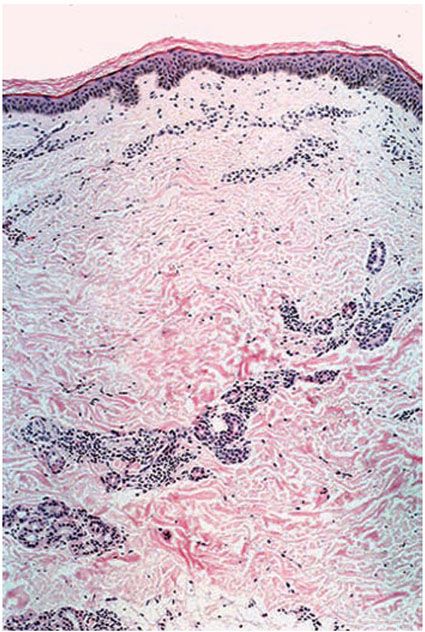
Figure 21-4 Indeterminate leprosy. Slight pandermal perineurovascular and periappendageal chronic inflammation (H&E stain).
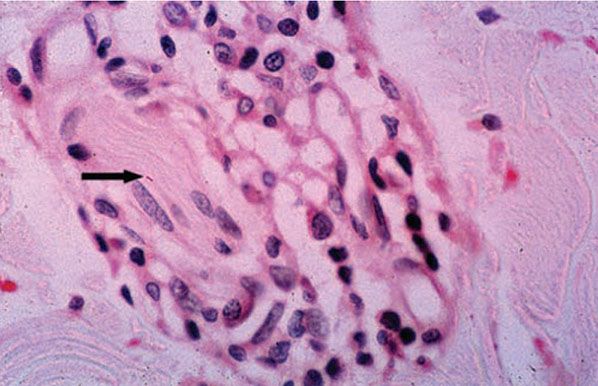
Figure 21-5 Indeterminate leprosy. High-power view of a small dermal nerve with surrounding lymphocytes and an intraneural acid-fast bacillus (arrow). There is no granuloma formation (Wade-Fite stain).
Lepromatous Leprosy
LL leprosy initially has cutaneous and mucosal lesions, with clinically manifest neural changes occurring later. The lesions usually are numerous and symmetrically arranged. There are three clinical types: macular, infiltrative-nodular, and diffuse. In the macular type, numerous ill-defined, confluent, either hypopigmented or erythematous macules are observed. They are frequently slightly infiltrated. The infiltrative-nodular type, the classical and most common variety, may develop from the macular type or arise as such. It is characterized by papules, nodules, and diffuse infiltrates that are often dull red. Involvement of the eyebrows and forehead often results in a leonine facies, with a loss of eyebrows and eyelashes. The lesions themselves are not notably hypoesthetic, although through involvement of the large peripheral nerves, disturbances of sensation and nerve paralyses develop. The nerves that are most commonly involved are the ulnar, radial, and common peroneal nerves.
The diffuse type of leprosy, called Lucio leprosy, which is most common in Mexico and Central America, shows diffuse infiltration of the skin without nodules. This infiltration may be quite inconspicuous except for the alopecia of the eyebrows and eyelashes it produces. Acral, symmetric anesthesia is generally present (54).
A distinctive variant of LL leprosy, the histoid type, first described in 1963 (55), is characterized by the occurrence of well-demarcated cutaneous and subcutaneous nodules resembling dermatofibromas.
Rarely, LL leprosy can present as a single lesion rather than as multiple lesions (56).
Histopathology. LL leprosy, in the usual macular or infiltrative-nodular lesions, exhibits an extensive cellular infiltrate that is almost invariably separated from the flattened epidermis by a narrow grenz zone of normal collagen ((Figs. 21-6 and 21-7). The macular lesions show a mild-to-moderate, superficial and deep, perivascular and periadnexal infiltrate of foamy histiocytes. The infiltrate may cause the destruction of the cutaneous appendages and extends into the subcutaneous fat. In florid lesions, the macrophages have abundant eosinophilic cytoplasm and contain a mixed population of solid and fragmented bacilli (BI = 4 or 5) (Fig. 21-4). The bacilli, on Wade-Fite staining, can be seen to measure about 5.0 by 0.5 μm and, if solid, may be packed like cigars. Bacilli are commonly observed in endothelial cells as well (Fig. 21-8). There is no macrophage activation to form epithelioid cell granulomas. Lymphocyte infiltration is not prominent, but there may be many plasma cells.
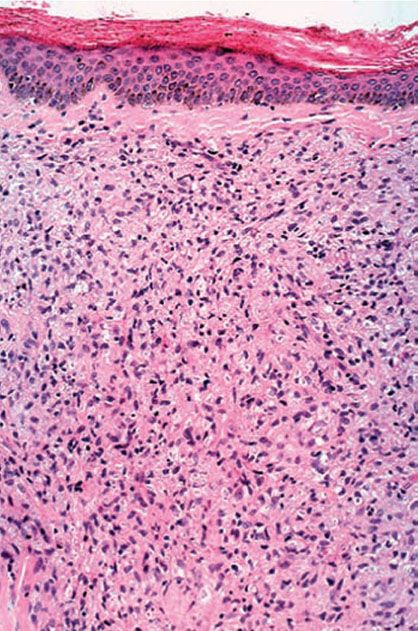
Figure 21-6 Lepromatous leprosy. Skin in multibacillary leprosy with a mass of macrophages in the dermis (no granuloma formation), leaving a clear grenz zone under the epidermis (H&E stain).
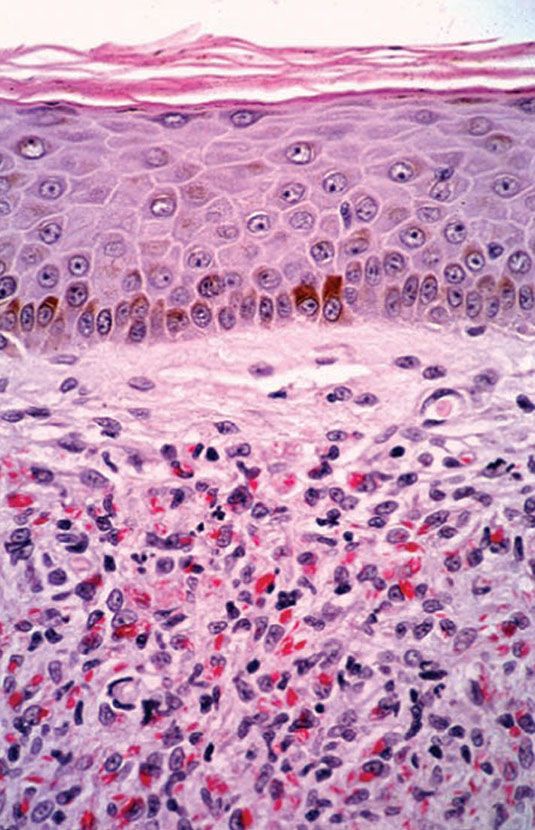
Figure 21-7 Lepromatous leprosy. Same case as Fig. 21-37. Acid-fast bacilli, mostly solid, in large numbers (Wade-Fite stain).
Figure 21-8 Lepromatous leprosy. Macrophages and endothelial cells of the capillary contain solid acid-fast bacilli (Wade-Fite stain).
In time, and with antimycobacterial chemotherapy, degenerate bacilli accumulate in the macrophages—the so-called lepra cells or Virchow cells—which then have foamy or vacuolated cytoplasm (Fig. 21-9). They resemble xanthoma cells and, on staining with fat stains, are shown to contain lipid—largely neutral fat and phospholipids—rather than cholesterol. The Wade-Fite stain reveals that the bacilli are fragmented or granular and, especially in very chronic lesions, disposed in large basophilic clumps called globi. In LL leprosy, in contrast to TT leprosy, the nerves in the skin may contain considerable numbers of leprosy bacilli but remain well preserved for a long time and slowly become fibrotic.
Figure 21-9 Lepromatous leprosy. Old, treated lesion, with large foamy macrophages and no identifiable bacilli (Wade-Fite stain).
When LL leprosy is treated, the bacilli die rapidly and become fragmented within weeks or months. However, it can take several years for the bacterial debris to be cleared by host macrophages. The M. leprae antigen may persist even longer and can be demonstrated by immunocytochemical stains (Wade-Fite or silver) even when no bacilli are evident (Fig. 21-10).
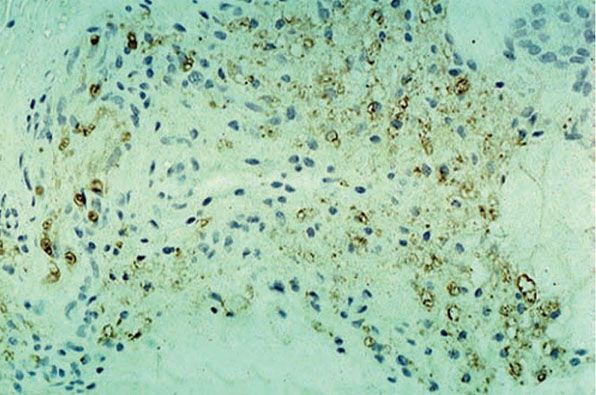
Figure 21-10 Lepromatous leprosy. Old, treated lesion; foamy macrophages remain but no acid-fast bacilli visible. However, an antimycobacterial immunocytochemical stain reveals persistent leprosy antigen (anti-BCG stain).
The histopathology of Lucio (diffuse) leprosy is similar but with a characteristic heavy bacillary infiltrate of the small blood vessels in the skin (54).
Histoid Leprosy
Histoid leprosy shows the highest loads of bacilli (frequently, the BI is 6), and the majority are solid staining, arranged in clumps like sheaves of wheat. The macrophage reaction is unusual in that the cells frequently become spindle shaped and oriented in a storiform pattern, similar to those of a fibrous histiocytoma (Fig. 21-11). The epidermis may be stretched over such dermal expansile nodules.
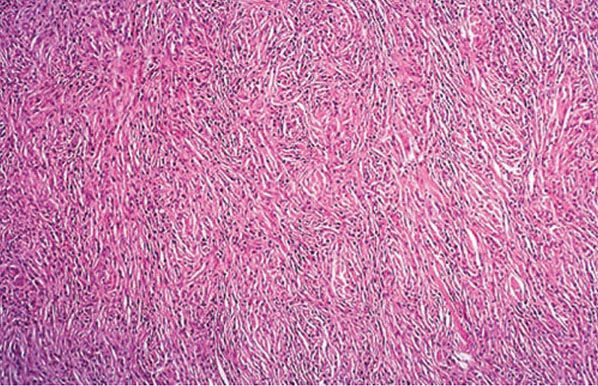
Figure 21-11 Lepromatous leprosy. A histoid lesion, with spindle cell proliferation of macrophages, resembling a storiform tumor. The acid-fast bacillus stain showed larger numbers of bacilli (H&E stain).
Borderline Lepromatous Leprosy
The lesions of BL leprosy are less numerous and less symmetrical than LL lesions and often display some central dimples.
Histopathology. The important difference between LL and BL leprosy histology is that in BL, the lymphocytes are more prominent and there is a tendency for some activation of macrophages to form poorly to moderately defined granulomas. Perineural fibroblast proliferation, forming an “onion skin” in cross section, is typical. Foamy cells are not prominent, and globi do not usually accumulate; the BI ranges from 4 to 5.
Midborderline Leprosy
In BB leprosy, the skin lesions are irregularly dispersed and shaped erythematous plaques with punched-out centers. There may be small satellite lesions. Edema is prominent in the lesions.
Histopathology. In BB leprosy, the macrophages are uniformly activated to epithelioid cells but are not focalized into distinct granulomas, and lymphocytes are scanty. There are no Langhans giant cells. The BI ranges from 3 to 4. Dermal edema is prominent between the inflammatory cells.
Borderline Tuberculoid Leprosy
In BT leprosy, the lesions are asymmetrical and may be scanty. They are dry, hairless plaques with central hypopigmentation. Nerve enlargement is usually found, and the lesions are usually anesthetic.
Histopathology. Granulomas with peripheral lymphocytes follow the neurovascular bundles and infiltrate sweat glands and erector pili muscles. Langhans giant cells are variable in number and are not large in size. Granulomas along the superficial vascular plexus are frequent, but they do not infiltrate up into the epidermis. Nerve erosion and obliteration are typical (Figs. 21-12 to 21-14). Acid-fast bacilli are scanty (BI ranges from 0 to 2) and most readily found in the Schwann cells of nerves. Immunocytochemical staining for S-100 protein often demonstrates the perineural and intraneural granuloma well Fig. 21-14B).
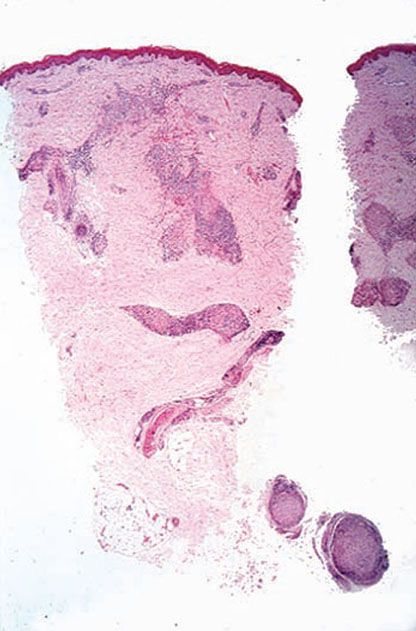
Figure 21-12 Tuberculoid leprosy. A typical lesion, showing pandermal perineurovascular granulomas and lymphocytes (H&E stain).
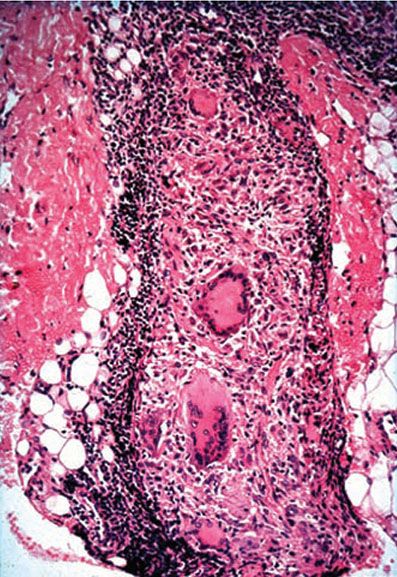
Figure 21-13 Tuberculoid leprosy. Granulomatous neuritis; dense lymphocytosis surrounding and eroding into the deep dermal nerve with giant cells (H&E stain).
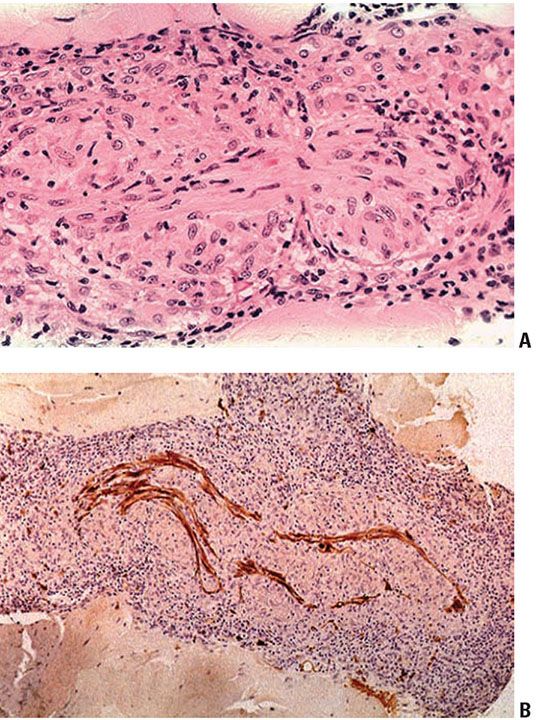
Figure 21-14 Tuberculoid leprosy. A: Typical endoneuritis with granulomas eroding into the nerve (H&E stain). B: Granulomatous neuritis; granulomas within the dermal nerve disrupting the Schwann cells and axons (S-100 immunoperoxidase stain).
Tuberculoid Leprosy
The skin lesions of TT leprosy are scanty, dry, erythematous, hypopigmented papules or plaques with sharply defined edges. Anesthesia is prominent (except on the face). The number of lesions ranges from one to five. Thickened local peripheral nerves may be found. The lesions heal rapidly on chemotherapy.
Histopathology. Primary TT leprosy has large epithelioid cells arranged in compact granulomas along with neurovascular bundles, with dense peripheral lymphocyte accumulation. Langhans giant cells are typically absent. Dermal nerves may be absent (obliterated) or surrounded and eroded by dense lymphocyte cuffs. Acid-fast bacilli are rarely found, even in nerves. A second pattern of TT leprosy is found in certain reactional states (see next page).
Peripheral Nerves
In all of these patterns of leprosy, the major peripheral nerves are often undergoing parallel pathologies. The inflammation is similar, and the same classification system is applied. However, the density of acid-fast bacilli is often a logarithm higher than in the nearby skin (57).
Leprosy Reactions
Leprosy reactions are classified into two main types (1 and 2). A third reaction is specific to Lucio multibacillary leprosy (58).
Type 1 Reactions
Because the immunopathologic spectrum of leprosy is a continuum, patients may move along it in both directions. Should such shifts be rapid, they induce an inflammatory reaction with edema that results in enlargement of lesions with more erythema (Fig. 21-15). Shifts toward the tuberculoid pole are called upgrading or reversal reactions; shifts toward the lepromatous pole are termed downgrading reactions. Both are aspects of delayed hypersensitivity, or type 1, leprosy reactions. TT patients are stable. BT patients may downgrade without treatment. Multibacillary patients, particularly those at the BL point, frequently upgrade on chemotherapy, and about one quarter of them will exhibit significant reactions. BB patients are most unstable and will move either way depending on therapy, often with reactions. The most important aspect of type 1 reactions is not the skin but the condition of the peripheral nerves, in which a similar inflammatory process is going on; reaction induces increased intraneural inflammation and edema, which is damaging. At worst, there is caseous necrosis of large peripheral nerves resulting from upgrading reactions.
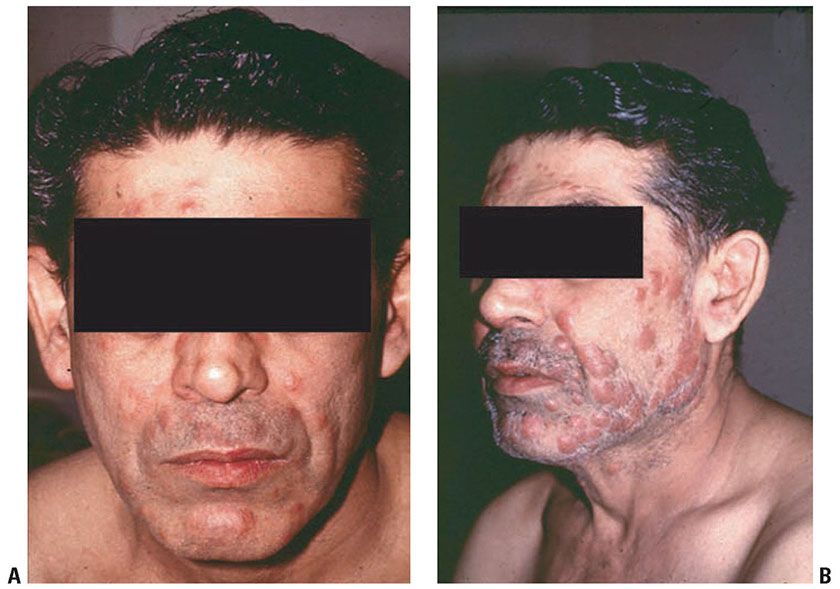
Figure 21-15 Leprosy reversal reaction. A: Pretreatment BL leprosy. B: Three months posttreatment, with enlargement of the skin lesions due to increased inflammation.
Histopathology. The histopathology of type 1 reactions has still not been well evaluated (59). The distinction between upgrading and downgrading reactions is difficult to make and may require serial examinations. Typically, there is edema within and about the granulomas and proliferation of fibrocytes in the dermis. In upgrading reactions, the granuloma becomes more epithelioid and activated, and Langhans giant cells are larger (Fig. 21-16); there may be erosion of granulomas into the lower epidermis, and there may be fibrinoid necrosis within granulomas and even within dermal nerves. In downgrading reactions, necrosis is much less common, and over time the density of bacilli increases. Multibacillary leprosy patients who upgrade on therapy show old foamy macrophages and degenerate bacilli admixed with newly developing epithelioid cell granulomas.
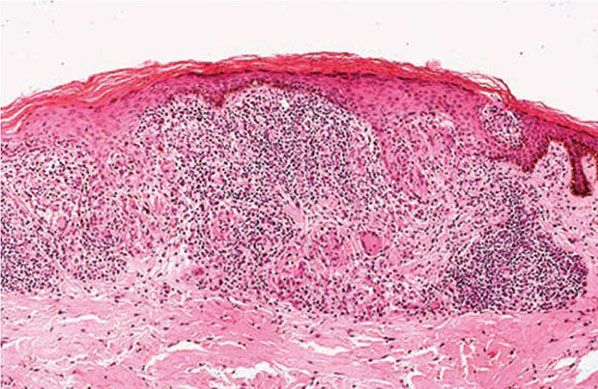
Figure 21-16 Leprosy: type 1 reaction (delayed hypersensitivity). Granulomatous erosion of the epidermis; this feature is not usually encountered in nonreacting leprosy (H&E stain).
Type 2 Reaction: Erythema Nodosum Leprosum
Erythema nodosum leprosum (ENL) occurs most commonly in LL leprosy and less frequently in BL leprosy. It may be observed not only in patients under treatment but also in untreated patients. Clinically, the reaction has a greater resemblance to erythema multiforme than to erythema nodosum. On the skin, tender red plaques and nodules, together with areas of erythema, and occasionally also purpura and vesicles, are observed. Ulceration, however, is rare. The eruption is widespread and accompanied by fever, malaise, arthralgia, and leukocytosis. New lesions appear for only a few days in some cases but for weeks and even years in others. This is the only type of reactional leprosy that responds to treatment with thalidomide.
Histopathology. In ENL, the lesions are foci of acute inflammation superimposed on chronic multibacillary leprosy (Fig. 21-17). Polymorph neutrophils may be scanty or so abundant as to form a dermal abscess with ulceration (60). Whereas foamy macrophages containing fragmented bacilli are usual, in some patients no bacilli remain and macrophages have a granular pink hue on Wade-Fite staining, indicating mycobacterial debris. An antimycobacterial immunocytochemical stain (e.g., anti-BCG) will indicate abundant antigen. A necrotizing vasculitis affecting arterioles, venules, and capillaries occurs in some cases of ENL; these patients may have superficial ulceration.

Figure 21-17 Leprosy: erythema nodosum leprosum (type 2 reaction). Macrophages with vacuoles and globi and a polymorphonuclear cell infiltrate (H&E stain).
Lucio Reaction
The Lucio reaction occurs exclusively in diffuse LL leprosy, in which it is a fairly common complication. It usually occurs in patients who have received either no treatment or inadequate treatment. In contrast to ENL, fever, tenderness, and leukocytosis are absent. The lesions consist of barely palpable, hemorrhagic, sharply marginated, irregular plaques. They develop into crusted lesions and, particularly on the legs, into ulcers. There may be repeated attacks or continuous appearance of new lesions for years. They are thought to result from immune complex deposition.
Histopathology. In the Lucio reaction, vascular changes are critical (48). Endothelial proliferation leading to luminal obliteration is observed in association with thrombosis in the medium-sized vessels of the dermis and subcutis. There is a sparse, largely mononuclear infiltrate. Dense aggregates of acid-fast bacilli are found in the walls and the endothelium of normal-appearing vessels as well as in vessels with proliferative changes (Fig. 21-18). Ischemic necrosis, brought on by the vascular occlusion, leads to hemorrhagic infarcts and results in crusted erosions or frank ulcers.
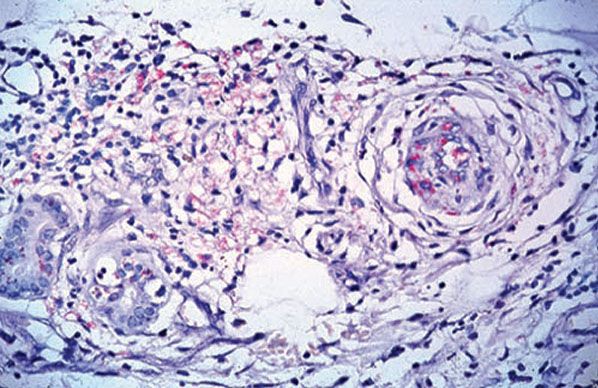
Figure 21-18 Leprosy, Lucio reaction. Dermal vessels showing endarteritis obliterans and abundant bacilli within endothelial cells (Wade-Fite stain).
Electron Microscopy of Leprosy
Under electron microscopy, M. leprae can be seen to consist of an electron-dense cytoplasm lined by a trilaminal plasma membrane. Outside of this membrane lies the bacterial cell wall surrounded by a radiolucent area, the waxy coating typical of mycobacteria (43). Lepra bacilli are found in the skin, predominantly in macrophages and in Schwann cells.
Pathogenesis of Leprosy
With respect to immunologic reactivity, patients with LL leprosy have a defect in cell-mediated immune responses to the lepra bacilli, which therefore cannot be eradicated from the body spontaneously (45,61,62). The primary defect lies in the T lymphocytes, which can be stimulated only slightly or not at all to react against the lepra bacilli and thus do not adequately activate macrophages to destroy phagocytosed bacilli. This defect is specific for M. leprae, because patients with LL leprosy show normal immunologic responses to antigens other than lepromin in both in vivo and in vitro testing.
The specific inability of T lymphocytes obtained from patients with LL leprosy to react against lepromin is shown by the fact that when these lymphocytes are incubated with lepromin, they show little or no production of macrophage migration-inhibiting factor (MIF). In contrast, the lymphocytes of patients with TT leprosy produce significant amounts of MIF on exposure to lepromin. One modern view is that in tuberculoid patients, exposure to leprosy antigens results in a predominant T helper 1 (TH1) cytokine secretion profile, which results in macrophage activation. Conversely, in lepromatous patients, the cytokine profile (TH2) inhibits cell-mediated immunity and promotes humoral immunity, which does not contribute to host defense (61,63). In reversal reactions, there is an increase in the lymphocyte response to lepromin during the reaction and a decrease during the postreaction phase.
Analysis of T-cell subsets in lesions has shown that in TT leprosy, with its high degree of resistance to the leprosy bacilli, the T-helper lymphocytes are distributed evenly throughout the epithelioid cell aggregates, and the suppressor T lymphocytes are restricted to the peripheries of the granulomas. In LL leprosy, both helper and suppressor T lymphocytes are distributed diffusely throughout the lesions (64). It is noteworthy that the distribution of helper and suppressor T cells in TT leprosy is similar to that observed in sarcoidosis.
In patients with either ENL or the Lucio reaction, deposits of IgG and the third component of complement (C3), as well as circulating immune complexes, have been found in the vessel walls of the dermal lesions. This suggests that both reactions are mediated by immune complexes (Gell and Coombes type III reaction) (52).
The lepromin skin test, or Mitsuda test, consists of the intradermal injection of a preparation of M. leprae derived from autoclaved infected human tissue. A positive reaction consists of the formation of a nodule measuring 5 mm in diameter or more after 2 to 4 weeks. On histologic examination, the nodule shows an epithelioid cell granuloma. The reaction is positive only in the high-resistance (TT and BT tuberculoid) forms of the disease. In indeterminate leprosy, it may be positive or negative. The test reveals the inability of patients at the lepromatous end of the spectrum to react to the injection of M. leprae with an epithelioid cell granuloma, and its main value is therefore as a marker of specific cell-mediated immunity to this organism, which varies continuously along the immunopathologic spectrum of HIV infection and leprosy.
Because HIV induces a generalized immunosuppressive state, and because a wide range of intracellular infectious agents normally controlled by the T-cell/macrophage system may proliferate to cause significant disease, it was expected that leprosy might also be affected. Specifically, the disease might become more prevalent in HIV and leprosy coendemic areas, and individual patients might downgrade toward lepromatous disease as their HIV disease progresses. However, epidemiologic studies have shown no effect of HIV infection on the incidence of leprosy in properly controlled studies (65,66), nor has a change in the proportions of tuberculoid versus lepromatous patients been noted (67). At present, the only clinicopathologic difference between HIV-infected and noninfected leprosy patients that is suggested is an increased likelihood of HIV-positive patients undergoing type 1 upgrading reactions (67,68). This rather paradoxical phenomenon awaits further evaluation.
Histopathologic Differential Diagnosis
The leprosy bacillus cannot yet be grown in vitro. TT (granulomatous) leprosy needs to be distinguished from the many other granulomatous dermatitides. The presence of acid-fast bacilli in nerves is conclusive proof of leprosy, as is the demonstration of an intraneural granuloma. The S-100 stain may highlight this phenomenon (69), although in practice, if the diagnosis is in doubt after investigation with ordinary stains, this immunocytochemical method is not usually diagnostic either. In leprosy, naked granulomas are found only in BB leprosy, and acid-fast bacilli will be found in the lesions. Sarcoidosis may rarely cause granulomas to form within peripheral nerves (70) but does not appear to do so in dermal nerves. The general vertical perineurovascular distribution of granulomatous inflammation and involvement of sweat glands in TT leprosy are helpful. The presence or absence of plasma cells or of intraepidermal lymphocytes is not helpful. Unlike other mycobacterial skin infections such as tuberculosis, and unlike granulomatous leishmaniasis, the epidermis in TT leprosy is usually flat and nonhyperplastic. Late secondary and tertiary cutaneous syphilis is characterized by epithelioid and giant cell granulomas in the dermis, not directly involving nerves, and the epithelium is usually hyperplastic. Intragranuloma necrosis (fibrinoid or caseating) occurs in leprosy in type 1 reactions, sometimes spontaneously; this can be confused with necrobiotic lesions such as granuloma annulare. Necrosis within a nerve that is granulomatous is diagnostic of leprosy.
Early, indeterminate leprosy overlaps with many specific and nonspecific dermatitides manifesting perineurovascular lymphocytic infiltrates. Finding bacilli in critical sites (in nerve, under the epidermis, in erector pili muscle, or in macrophages) is critical. In the absence of bacilli and the presence of a pandermal infiltrate, leprosy can only be suspected. Problems may arise from contaminant mycobacteria in staining solutions and in the water baths used for floating out sections. Such organisms are usually above the plane of the section, overlap the cell nuclei, and usually stain darker than M. leprae. The use of PCR (polymerase chain reaction) for identifying paucibacillary leprosy in skin sections and tissues has not been as successful as it once was thought to be (71).
Ultimately, there is a proportion of suspect paucibacillary leprosy lesions for which the histopathologist cannot make a firm diagnosis either way, and intra- and interobserver variation may be considerable (72). Clinical diagnosis of single-lesion leprosy is also imperfect (56).
LL leprosy infiltrates can resemble xanthoma, although the cytoplasmic granularity is coarser in the latter disease. The presence of acid-fast bacilli is obviously important, and in long-treated lesions that may cause confusion, antimycobacterial immunocytochemistry is helpful. ENL may be overlooked because it is a combined chronic and acute inflammatory infiltrate, but once thought of, the presence of bacilli or antigen is diagnostic. Certain other mycobacterioses in immunosuppressed patients, such as M. avium complex, may produce histoid-like multibacillary lesions (73) (see Figs. 21-29 to 21-31); however, nerves are not involved in this infection.
Principles of Management
Multidrug therapy is essential to prevent development of resistance. Common regimens include dapsone plus rifampin for TT leprosy and clofazimine may be added to these for LL leprosy, for 6 and 12 months, respectively. Treatment algorithms depend somewhat on the burden of disease, and all patients with a diagnosis of leprosy should be treated in conjunction with an infectious disease specialist. Neuritis and immunologic reactions may be treated with corticosteroids with or without other anti-inflammatory agents. Type 1 reactions may be observed in HIV patients after initiation of antiretroviral therapy. The response to treatment appears to be comparable among HIV-positive and HIV-negative individuals (51).
TUBERCULOSIS
Tuberculosis remains a dominant public health problem in resource-poor countries and has reemerged in the remaining countries around the world due to a global resurgence. Factors leading to the increased incidence of tuberculosis include immigration from endemic countries (particularly in Asia and Africa), increased movement of refugees, the HIV pandemic, and poverty (74). As a result, cutaneous tuberculosis remains a clinical and diagnostic problem (75,76). It is estimated that cutaneous tuberculosis accounts for a small proportion of all tuberculosis infections (1% to 2%), but given the high prevalence of tuberculosis in some areas, the absolute number of cases in not insignificant (77).
Infection of the skin and subcutis by M. tuberculosis occurs by three routes: (a) by direct inoculation into the skin (causing a primary chancre, or tuberculosis verrucosa cutis (TVC), or tuberculosis cutis orificialis lesions); (b) by hematogenous spread from an internal lesion (causing lupus vulgaris, miliary tuberculosis, and tuberculous gumma lesions); and (c) from an underlying tuberculous lymph node by direct extension (causing scrofuloderma). For descriptive purposes, these different tuberculous dermatitides are delineated (a modified “Beyt classification” is used) (40). But in clinical practice, many cases do not readily fit into these clinical and histologic categories (41,78,79). Scrofuloderma and lupus vulgaris appear to be the most common forms (77). In a study of cutaneous tuberculosis infections in a pediatric population from India, with histopathologic evaluation, there was a large spectrum of clinical patterns. The different patterns of cutaneous tuberculosis seen were scrofuloderma (37%), lichen scrofulosorum (33%), lupus vulgaris (21%), TVC (4%), papulonecrotic tuberculid (4%), and erythema nodosum (3%). Systemic associations were seen in 53%, namely tuberculosis lymphadenitis (30%), pulmonary tuberculosis (13%), abdominal tuberculosis (6%), and tuberculosis arthritis (6%) (80).
The basic reaction of human tissues to tuberculous bacilli is a sequence, first of acute nonspecific inflammation during which the bacilli multiply and cannot be phagocytosed and killed by neutrophil polymorphs. Macrophages then phagocytose the organisms, but their efficacy in killing them depends crucially on enhanced activation. T-cell-mediated allergy to tuberculous antigens induces secretion of cytokines that recruit and activate macrophages, which become epithelioid cells. Some will fuse to form giant cells, the typical form of which in tuberculosis is the Langhans giant cell with the nuclei arranged around the periphery of the cytoplasm. As delayed hypersensitivity increases, there is caseation necrosis within the granuloma, caseation being a homogeneous eosinophilic infarct-like necrotic process whereby the macrophages die. It is probably mediated by cytokines (such as tumor necrosis factor [TNF]) and the macrophage proteases. This necrotic process inactivates or kills many of the mycobacteria in the lesion, but it does not eliminate them (81,82).
The necrotic granuloma is thus typical of tuberculosis and other mycobacterial infections, but it is not specific, being seen in numerous other infections that involve this type of cell-mediated immunity (e.g., histoplasmosis, syphilis, and leishmaniasis).
M. tuberculosis is an obligate aerobe, and thrives at a pO2 of 140 mm Hg, which is near the pO2 of atmospheric air. This is why in conditions like scrofuloderma, where the lesion is exposed to the outside air, bacterial growth, and consequent detection on smears and sections, is much higher than in other forms of cutaneous tuberculosis.
The determinants of what happens in tuberculosis infection therefore include the virulence of the organism (tuberculosis is more virulent than most of the nontuberculosis mycobacteria), the size of the inoculum, the route of infection, and the immune status of the patient. It is evident that if cell-mediated immunity is impaired, the T-cell/macrophage system will not operate to contain the infection. The resulting pathology is generally less granulomatous (fewer activated macrophages) and has a higher density of mycobacteria. Such conditions are HIV infection with its sequel of AIDS, steroid therapy, and cytotoxic drugs. The concept of bacterial load in cutaneous tuberculosis has been proposed (in analogous manner to that described for M. leprae in Hansen disease). Accordingly, cutaneous tuberculosis can be subclassified into multibacillary forms (primary inoculation tuberculosis, scrofuloderma, tuberculosis periorificialis, acute miliary tuberculosis, and gumma) and paucibacillary forms (TVC, occasional forms of lupus vulgaris, papulonecrotic tuberculid, erythema induratum of Bazin, and lichen scrofulosorum) (77).
MULTIBACILLARY FORMS
In the multibacillary forms of tuberculosis, numerous mycobacteria can be frequently demonstrated using the Ziehl–Neelsen technique and are more readily cultured (83).
Primary Tuberculosis
Clinical Summary. Primary infection with tuberculosis occurs only rarely on the skin. Children or adults may acquire it following minor trauma or contact with infected material—as a result, for instance, of mouth-to-mouth artificial respiration (84), inoculation during an autopsy (85), needle-stick injury (86), or inoculation during tattooing (87). Not surprisingly, this form of cutaneous tuberculosis is frequent in health care and laboratory personnel. Affected children are usually infected through exposure to a household member or caregiver with active pulmonary tuberculosis. The most commonly affected sites are face, hands, and feet. In countries where tuberculosis is endemic, such as India, some patients develop primary (or more often secondary) infection on the soles of the feet, from walking barefoot over expectorated tuberculosis. Primary gingivitis, granulomatous paronychia, and penile chancre are considered variants of this form. The primary lesion is typically a papule or nodule which eventually ulcerates (77). Usually, the fully developed cutaneous lesion arises within 2 to 4 weeks after the inoculation. It consists of an asymptomatic crust-covered ulcer referred to as tuberculous chancre. The regional lymph nodes become enlarged and tender and may suppurate and produce draining sinuses.
Histopathology. The histologic development of the lesion is very much like that observed in experimental cutaneous inoculation of the guinea pig. In the earliest phase, the histologic picture is that of an acute neutrophilic reaction, with areas of necrosis resulting in ulceration. Numerous tubercle bacilli are present, particularly in the areas of necrosis (85). After 2 weeks, monocytes and macrophages predominate. Three to 6 weeks after onset, epithelioid cells and giant cell granulomas develop, followed by caseation necrosis within the granuloma (Fig. 21-19). In time, the necrosis lessens, and the number of tubercle bacilli decreases until it is so greatly reduced that the bacilli may be impossible to demonstrate in histologic sections. Simultaneous with the decrease in the number of tubercle bacilli in the lesion, the tuberculin test with purified protein derivative (PPD), previously negative, becomes positive. The draining lymph nodes receive tubercle bacilli and enlarge through developing caseating granulomas, parallel to a primary infection in the lung. Of note, primary cutaneous tuberculosis only rarely evolves into disseminated disease.
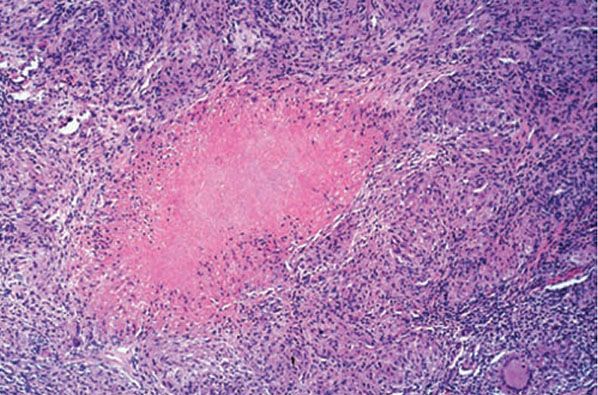
Figure 21-19 Tuberculosis. A prosector’s wart following inoculation of the finger from a tuberculous cadaver. There is central caseation necrosis with dense macrophage and lymphocyte surround (H&E stain).
Scrofuloderma
Clinical Summary. Scrofuloderma is the most common form of cutaneous tuberculosis in developing countries, and some series from Europe. It represents a direct extension to the skin of an underlying tuberculous infection present most commonly in a lymph node, and less commonly in joints or a bone. The lesion first manifests itself as a blue-red, painless swelling that breaks open and then forms an ulcer with irregular, undermined, blue borders. The ulcer is frequently surrounded by keloidal cicatricial tissue. The axillae, neck, chest wall, and inguinal region are most commonly affected. Patients typically have active pulmonary or pleural tuberculosis (77).
Histopathology. The center of the lesion usually exhibits nonspecific changes, such as abscess formation or ulceration. In the deeper portions and at the periphery of the lesion, if the biopsy specimen is adequate, tuberculoid granulomas with a considerable amount of necrosis and a pronounced inflammatory reaction usually are seen. Often, the number of tubercle bacilli is sufficient for them to be found in acid-fast stained histologic sections. The PPD is typically strongly positive.
Tuberculosis Cutis Orificialis
Clinical Summary. This form of cutaneous tuberculosis is rare. The lesions of tuberculosis cutis orificialis (periorificial tuberculosis) are shallow, painful ulcers (in contrast to mucocutaneous leishmaniasis and rhinoscleroma, which are typically nonpainful) with a granulating base occurring singly or in small numbers on or near the mucosal orifices of patients with advanced internal tuberculosis. The infection has spread by direct contamination from an internal lesion that is excreting bacilli. Most patients have a low degree of immunity. The ulcers, which are often very tender, may occur inside the mouth, on the lips, around the anus, or on the perineum (88). Anal lesions must be differentiated from malignancy. In the case of genitourinary tuberculosis, ulcers may occur on the vulva.
Histopathology. The histologic picture may show merely an ulcer surrounded by a nonspecific inflammatory infiltrate. In most instances, tuberculoid granulomas with pronounced necrosis are found deep in the dermis. Tubercle bacilli are usually readily demonstrated in the sections, even when the histologic appearance is nonspecific.
Acute Miliary Tuberculosis
Clinical Summary. Involvement of the skin with miliary tuberculosis is rare in the immunocompetent, occurring mostly in children and adolescents and only occasionally in adults. Usually, internal involvement is widespread as a result of hematogenous dissemination, and the cutaneous eruption is generalized, consisting of erythematous papules and pustules 2 to 5 mm in diameter (89). The tuberculin test generally is negative. The disease has a high fatality rate.
However, a milder form of hematogenous dissemination of tubercle bacilli exists in neonates born of tuberculous mothers. This form shows limited visceral involvement and only a few scattered erythematous papules with central crusts (90).
Histopathology. In severe cases, the center of the papule shows a microabscess containing neutrophils, cellular debris, and numerous tubercle bacilli. This is surrounded by a zone of macrophages with occasional giant cells. In the milder form, the histologic picture in the skin is similar, except that the Ziehl–Neelsen stain is negative for acid-fast organisms.
Tuberculous Gumma
Clinical Summary. Tuberculous gummas are cold abscesses typically occurring on the trunk or extremities of patients with no involvement of the underlying tissue. They are thought to represent hematogenous infection of the skin from an internal lesion that remains latent, and under the appropriate conditions (malnutrition, immunosuppression) may result in a large dermal or subcutaneous nodule that is necrotic and ultimately ulcerates the epidermis.
Histopathology. Most of the lesion is caseation necrosis with a rim of epithelioid cells and giant cells (Figs. 21-20 and 21-21). Acid-fast bacilli are scanty, but usually demonstrable on histologic sections.
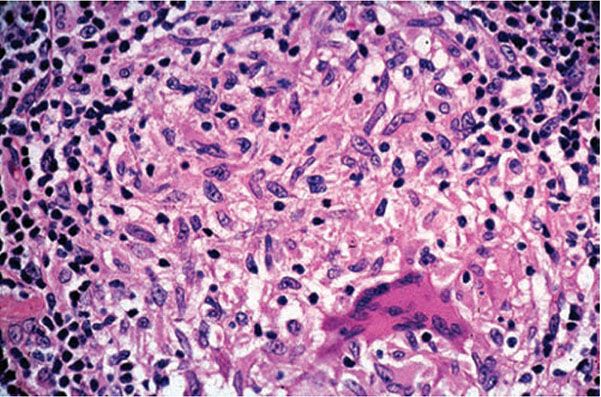
Figure 21-20 Lupus vulgaris. Same lesion as in Fig. 21-24, showing an epithelioid cell granuloma and Langhans giant cell (H&E stain).
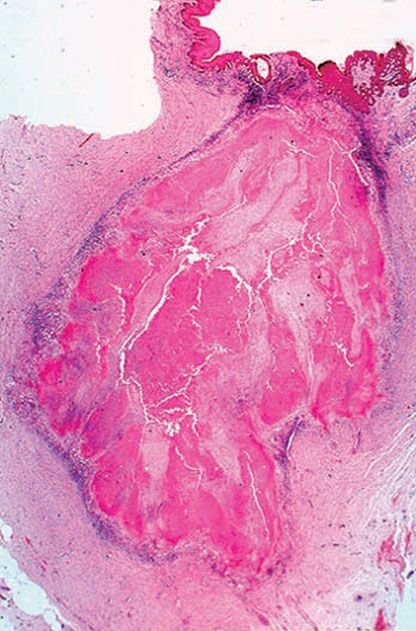
Figure 21-21 Tuberculosis. A tuberculoma-like appearance with much caseation necrosis in the dermis (H&E stain).
PAUCIBACILLARY FORMS
In the paucibacillary forms, mycobacteria may be difficult or impossible to detect using the Ziehl–Neelsen technique and are less readily cultured (83).
Tuberculosis Verrucosa Cutis
Clinical Summary. TVC represents an inoculated exogenous infection of the skin in persons with a degree of immunity—that is, previous exposure to tuberculosis. In TVC, a single, painless verrucous plaque with an inflammatory border and showing gradual peripheral extension is usually observed. The verrucous surface exhibits fissures from which pus often can be expressed. The most common sites are the hands and, in children, the knees, buttocks, thighs, and feet.
Histopathology. The characteristic histologic picture is that of pseudocarcinomatous squamous hyperplasia with marked hyperkeratosis and acanthosis. Beneath the epidermis, there is often a mixed lymphohistiocytic infiltrate with an occasional sprinkling of neutrophils. This may have a lichenoid appearance. Abscess formation may be observed in the upper dermis or within downward extensions of the epidermis. In the mid-dermis or upper dermis, tuberculoid granulomas are usually present, though often multiple step sections need to be cut before a granuloma is found (Fig. 21-22). Identification of tuberculous bacilli and/or their isolation from culture is the exception rather than the rule. Lesions tend to chronicity but may remain sensitive to antituberculous treatment, which in itself may function as a diagnostic aid (77).
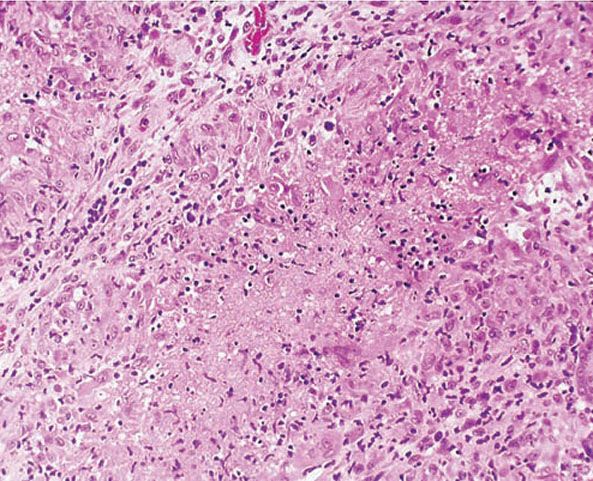
Figure 21-22 Tuberculosis. An epithelioid cell granuloma and caseation (H&E stain).
Lupus Vulgaris
Clinical Summary. Lupus, which translates to “wolf” in Latin, was initially used to refer to any ulcerative lesion resembling a wolf bite. In 1808, Willan used the word lupus for the first time to describe the late stages of facial cutaneous tuberculosis in his book “On Cutaneous Diseases.” Lupus vulgaris is the most common form of cutaneous tuberculosis in India and Pakistan, and it used to be the predominant form encountered in Europe. The lesions of lupus vulgaris are usually found on the face. The skin of and around the nose is frequently involved (91). The primary lesion consists of one or a few well-demarcated, reddish-brown patches containing deep-seated nodules, each about 1 mm in diameter, known as “lupomes.” If the blood is pressed out of the skin with a glass slide (diascopy), these nodules stand out clearly as yellow-brown macules, referred to, because of their color, as apple-jelly nodules. The characteristic fully developed lesion is a slowly enlarging plaque with a slightly elevated, verruciform border and central atrophy. It is a characteristic feature of lupus vulgaris that new lesions may appear in areas of atrophy, particularly after temporal immunosupression. Superficial ulceration or verrucous thickening of the skin occurs occasionally. Squamous cell carcinoma develops at the margins of ulcers in rare instances.
Histopathology. Tuberculoid granulomas composed of epithelioid cells and giant cells are present. Caseation necrosis within the tubercles is slight or absent (92). Although the giant cells usually are of the Langhans type, with peripheral arrangement of the nuclei, some can be of the foreign body type, with irregular arrangement of the nuclei. There is an associated infiltrate of lymphocytes (Figs. 21-23 and 21-24). Sometimes, this may be so prominent that the granulomatous component is obscured. This is often seen in TVC-like lesions. Tuberculoid granulomas cause destruction of the cutaneous appendages. In areas of healing, extensive fibrosis may be present.
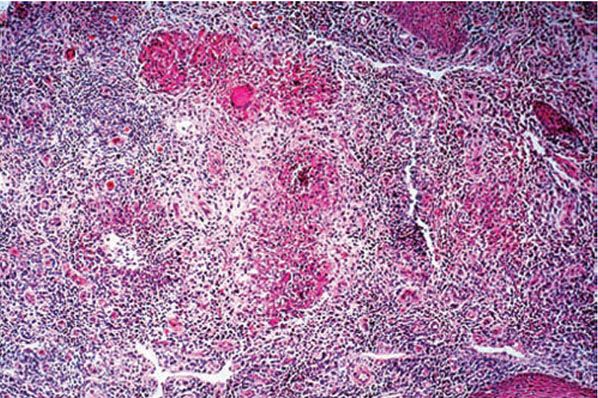
Figure 21-23 Tuberculosis. Epithelioid cell granulomas with central acute inflammation (“mixed” granuloma). Scanty acid-fast bacilli were seen in this case (H&E stain).
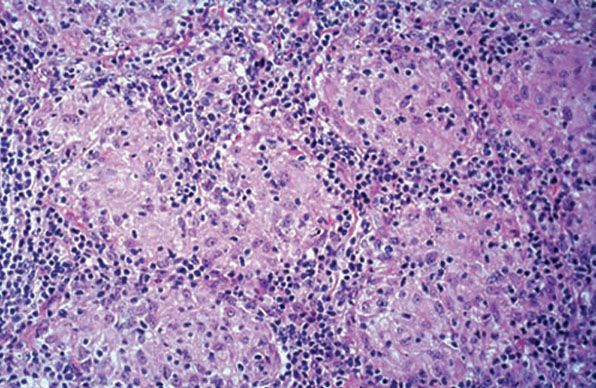
Figure 21-24 Lupus vulgaris. Near-confluent nonnecrotizing epithelioid cell granulomas in the dermis (H&E stain).
Secondary changes in the epidermis are common. The epidermis may undergo atrophy and subsequent destruction, causing ulceration, or it may become hyperplastic, showing acanthosis, hyperkeratosis, and papillomatosis. At the margins of ulcers, pseudoepitheliomatous hyperplasia often exists. Unless a deep biopsy is done in such cases, only the epithelial hyperplasia and a nonspecific inflammation may be seen, and the diagnosis may be missed. In rare instances, squamous cell carcinoma supervenes (93).
Tubercle bacilli are present in such small numbers that they can very rarely be demonstrated by staining methods or mycobacterial culture. Single case reports and small series have suggested that PCR detection of mycobacterial DNA is more often positive (94). However, the difference in sensitivity when comparing PCR to histopathologic sections stained with the Ziehl–Neelsen technique was only marginal in the largest series (79.4% vs. 73.5%, respectively) and not statistically significant (95). In some instances of lupus vulgaris, when an old focus of primary infection cannot be detected, a positive tuberculin test and the response to antituberculous therapy must suffice as proof of a tuberculous etiology.
Pathogenesis. Lupus vulgaris is a form of secondary or reactivation tuberculosis developing in previously infected and sensitized persons. Hypersensitivity to PPD tuberculin is high. Although the mode of infection often is not apparent, the disease rarely seems to be the result of an exogenous reinfection of the skin; usually it results from hematogenous spread from an old, reactivated focus in the lung or from lymphatic extension from a tuberculous cervical lymphadenitis (40).
Tuberculids
The tuberculids, a term first proposed in 1896 (96), is traditionally used to denote three entities: papulonecrotic tuberculid, lichen scrofulosorum, and erythema induratum of Bazin. (Bazin disease, nodular vasculitis; see Chapters 8 and 20). More recently, the term nodular tuberculid has been proposed for a rare subset of patients with nonulcerating nodules in the lower extremities, in whom the histopathologic changes are located in the dermis and the subcutaneous fat, thus representing a hybrid between papulonecrotic tuberculid and erythema induratum of Bazin (97,98).
Tuberculids are thought to represent a cutaneous immunologic reactions in patients with tuberculosis, often occult, elsewhere in the body. The most common sites of infection are lymph nodes (99). By definition, stains for acid-fast bacilli and culture for mycobacteria are negative; delayed hypersensitivity skin tests for tuberculosis are positive, and the lesions heal on antituberculous therapy. Recently, using the PCR technique, mycobacterial DNA has been identified in some lesions (100). There is controversy as to whether tuberculids represent immunologic reactions to degenerate dead bacilli or antigenic fragments thereof that have been deposited in the skin and subcutis, or true paucibacillary infections where bacilli are extremely difficult to detect (77).
Papulonecrotic Tuberculid
Clinical Summary. Papular necrotic tuberculid (papulonecrotic tuberculid) is believed to represent a paucibacillary form of hematogenous spread of tuberculosis. Primary lesions consist of 1- to 5-mm erythematous papules with an umbilicated necrotic center that usually develop on the extensor region of the limbs in a symmetrical distribution (101). The lower abdomen, trunk, buttocks, and earlobes can also be affected. The ulcers heal, leaving varioliform, depressed scars. Children and young adults with active tuberculosis elsewhere are most commonly affected. Recurrences may be observed until treated. Occasionally, they may develop into lupus vulgaris.
Histopathology. Vascular involvement is observed in early lesions. It may consist of a leukocytoclastic vasculitis or a lymphocytic vasculitis. In either case, it is associated with fibrinoid necrosis and thrombotic occlusion of individual vessels (102). Subsequently, a wedge-shaped area of necrosis forms, with its broad base toward the epidermis (101) (Figs. 21-25 and 21-26). As this wedge is gradually cast off, epithelioid and giant cells gather around its periphery, although focal granuloma formation is poor. Follicular necrosis or suppuration may occur, although diffuse acute inflammatory cells are infrequent. Ziehl–Neelsen stains are, of course, negative.
Differential Diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








