Introduction548
INTRODUCTION
SUPERFICIAL PYOGENIC INFECTIONS
IMPETIGO
Common impetigo
Bullous impetigo
Histopathology
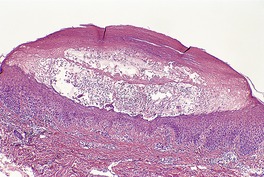
Fig. 23.1
STAPHYLOCOCCAL ‘SCALDED SKIN’ SYNDROME (SSSS)
Staphylococcal epidermolytic toxin syndrome
Histopathology54
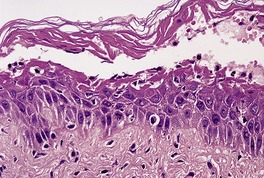
Fig. 23.2
STAPHYLOCOCCAL TOXIC SHOCK SYNDROME
Histopathology61
STREPTOCOCCAL TOXIC SHOCK SYNDROME
Histopathology
PERIANAL STREPTOCOCCAL DERMATITIS
ECTHYMA
Histopathology
DEEP PYOGENIC INFECTIONS (CELLULITIS)
ERYSIPELAS
Histopathology
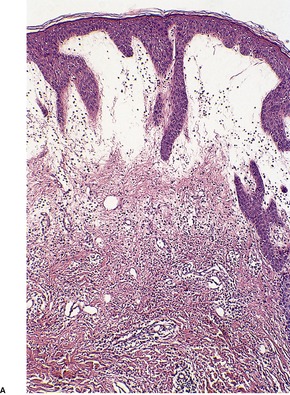
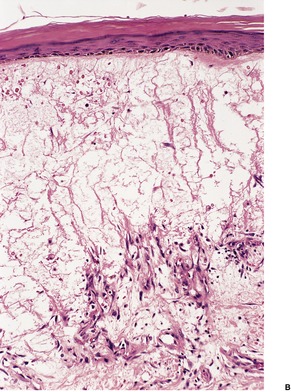
Fig. 23.3
ERYSIPELOID
Histopathology168
BLISTERING DISTAL DACTYLITIS
CELLULITIS
NECROTIZING FASCIITIS
Histopathology207. and 208.
MISCELLANEOUS SYNDROMES
Clostridial myonecrosis (gas gangrene)
Progressive bacterial synergistic gangrene
Erosive pustular dermatosis
Histopathology
BLASTOMYCOSIS-LIKE PYODERMA
Histopathology
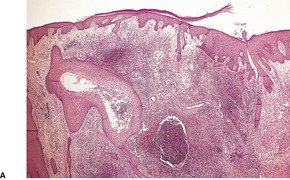
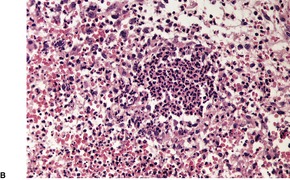
Fig. 23.4
CORYNEBACTERIAL INFECTIONS
DIPHTHERIA
Histopathology
ERYTHRASMA
Histopathology
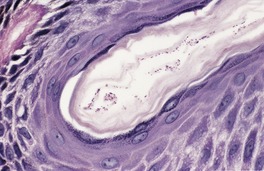
Fig. 23.5
Electron microscopy
TRICHOMYCOSIS
PITTED KERATOLYSIS
Histopathology306. and 314.
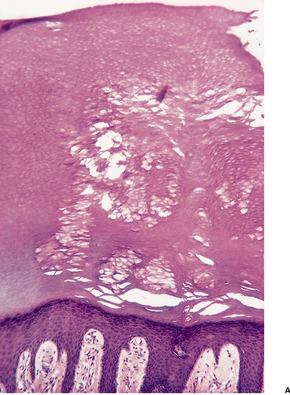
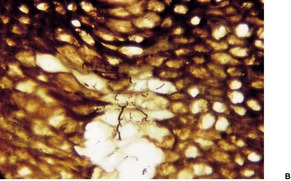
Fig. 23.6
NEISSERIAL INFECTIONS
MENINGOCOCCAL INFECTIONS
Histopathology
GONOCOCCAL INFECTIONS
Histopathology
MYCOBACTERIAL INFECTIONS
TUBERCULOSIS
Classification
Primary tuberculosis
Histopathology393
Lupus vulgaris
Histopathology425
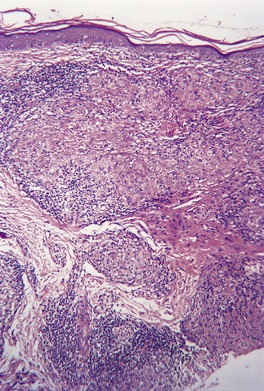
Fig. 23.7
Tuberculosis verrucosa
Scrofuloderma
Histopathology332
Orificial tuberculosis
Histopathology
Disseminated cutaneous tuberculosis
Histopathology502
Tuberculids
Histopathology
Treatment of cutaneous tuberculosis
INFECTIONS BY NON-TUBERCULOUS (ATYPICAL) MYCOBACTERIA
Mycobacterium ulcerans infection (Buruli ulcer)
Histopathology568. and 580.
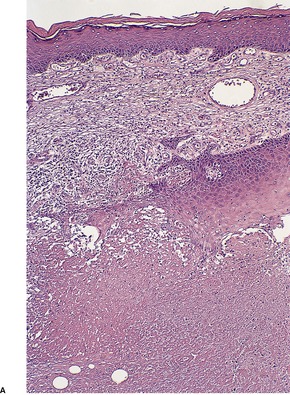
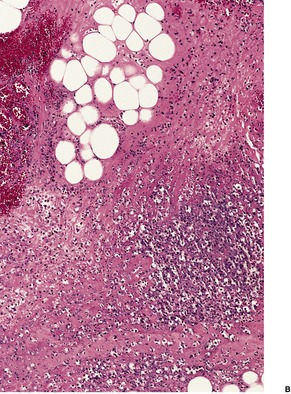
Fig. 23.8
Mycobacterium marinum infection (swimming pool granuloma)
Histopathology605.606. and 607.
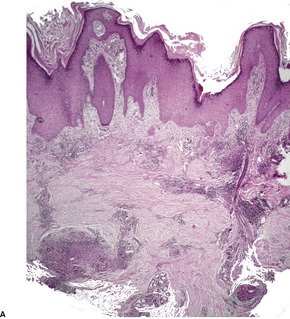
Fig. 23.9
Other non-tuberculous (atypical) mycobacteria
Treatment of non-tuberculous mycobacteria
Histopathology330
LEPROSY
Clinical classification of leprosy
Reactional states in leprosy735
Treatment of leprosy
Histopathology735. and 753.
Fig. 23.10
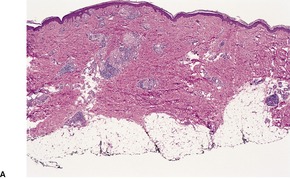
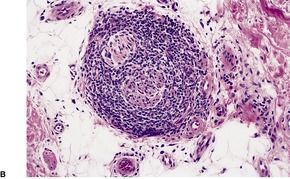
Fig. 23.11
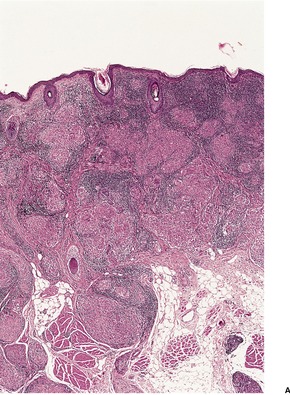
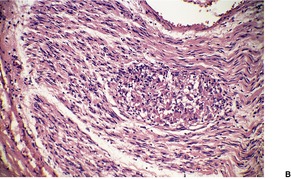
Fig. 23.12
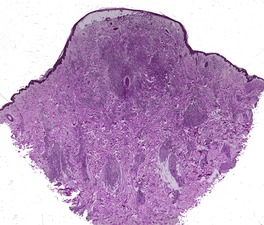
Fig. 23.13
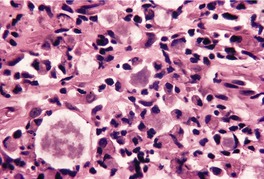
Fig. 23.14
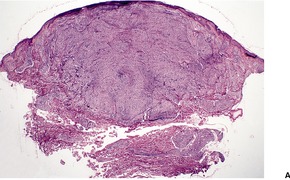
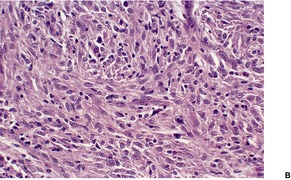
Fig. 23.15
MISCELLANEOUS BACTERIAL INFECTIONS
ANTHRAX
Histopathology844.845. and 857.
BRUCELLOSIS
Histopathology858. and 859.
YERSINIOSIS
Histopathology
GRANULOMA INGUINALE
Histopathology868. and 874.
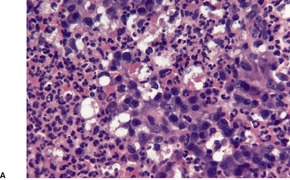
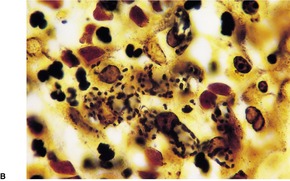
Fig. 23.16
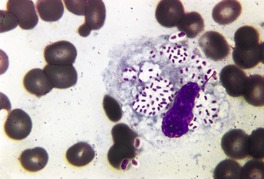
Fig. 23.17
Electron microscopy
CHANCROID
Histopathology878. and 890.
RHINOSCLEROMA
Histopathology
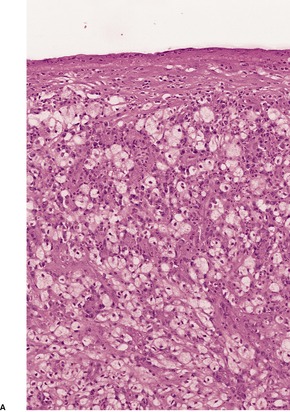
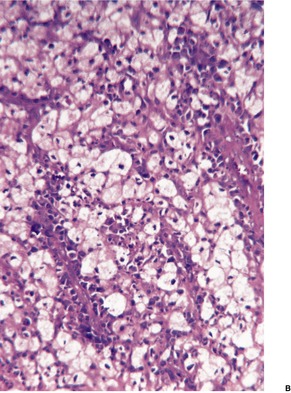
Fig. 23.18
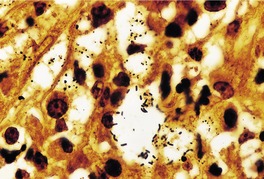
Fig. 23.19
Electron microscopy
TULAREMIA
Histopathology
LISTERIOSIS
Histopathology
CAT-SCRATCH DISEASE
Histopathology916. and 932.
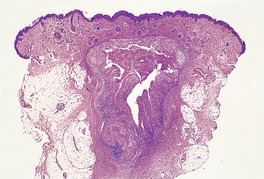
Fig. 23.20
MALAKOPLAKIA
Histopathology935
‘SAGO PALM’ DISEASE
Histopathology
CHLAMYDIAL INFECTIONS
PSITTACOSIS
LYMPHOGRANULOMA VENEREUM
Histopathology
RICKETTSIAL INFECTIONS
Disease
Organism
Mode of transmission
Rocky Mountain spotted fever
R. rickettsii
Tick
Boutonneuse fever
R. conorii
Tick
African tick bite fever
R. africae
Tick
Rickettsialpox
R. akari
Mite
Siberian tick typhus
R. sibirica
Tick
Queensland tick typhus
R. australis
Tick
Epidemic typhus
R. prowazakii
Louse feces
Murine typhus
R. mooseri (typhi)
Flea feces
Scrub typhus
O. tsutsugamushi
Mite
Q fever
Coxiella burnetii
Aerosol
Histopathology
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



Bacterial and rickettsial infections
Superficial pyogenic infections548
Deep pyogenic infections (cellulitis)551
Rickettsial infections572
Various bacteria form part of the normal resident flora of the skin. In the past, these organisms were regarded as symbiotic, but there is emerging evidence that these organisms may protect the host; as such they are mutualistic rather than symbiotic. 1 This subject has been reviewed recently (2008). 1 In certain circumstances some of these bacteria may assume pathogenic importance. Other bacteria are present only in pathological circumstances. In this chapter the following categories of bacterial infections will be considered: pyogenic, corynebacterial, neisserial, mycobacterial, miscellaneous, chlamydial, and rickettsial. Pyogenic infections, usually caused by Staphylococcus aureus and strains of Streptococcus, are numerically the most important bacterial infections of the skin. Staph. aureus-mediated skin infections require the adherence of the organism to the epidermis, if the skin surface is intact. Following adherence, the organism then invades keratinocytes, resulting in cytokine production and release. 2 It has recently been found to induce the expression of tumor necrosis factor-α (TNF-α) in keratinocytes. 3 Even exfoliative toxin-negative strains of Staph. aureus possess exotoxins capable of disrupting the barrier function of tight junction proteins. 4 Two distinct groups of pyogenic infection (superficial and deep) can be distinguished on the basis of the anatomical level of involvement of the skin. The pyogenic infections, with the exception of the staphylococcal ‘scalded skin’ syndrome, which results from the effects of a bacterial exotoxin, are characterized histologically by a heavy infiltrate of neutrophils. These organisms may also infect hair follicles, resulting in folliculitis. Furuncles (see p. 404) are deep-seated acute infections based on the pilosebaceous unit and adjacent dermis. They are of interest because of their association with Staph. aureus expressing the Panton–Valentine leukocidin genes. 5 This strain is found in 42% of isolates from furuncles, and in all cases of epidemic furunculosis. 5
Corynebacterial infections, with the exception of diphtheria, are usually limited to the stratum corneum and, as a consequence, there is no significant inflammatory response: at first glance, the biopsy may appear normal.
Neisserial infections of the skin are rare, although they are an important cause of urethritis. Cutaneous lesions may occur in neisserial septicemias.
Mycobacterial infections usually result in a granulomatous tissue reaction, but this depends on the immune status of the individual, including the development of delayed hypersensitivity. Exceptions include lepromatous leprosy, in which a histiocytic response occurs, and some infections by atypical mycobacteria, in which suppurative granulomas, suppuration, and even non-specific chronic inflammation may result at various times.
A variety of inflammatory reactions can be seen in the group of miscellaneous bacterial infections of the skin. The chapter closes with a brief discussion of chlamydial infections and rickettsial infections. Each group will be discussed in turn.
Although they are bacteria, infections by the actinomycetes are considered in Chapter 25 (pp. 600–602) because they produce lesions that are clinicopathologically similar in many respects to those produced by some fungi (mycetomas).
Although there are many facets to the defense mechanisms that the body has to various microorganisms, there has been recent interest in toll-like receptors (TLRs) as essential components of the innate immune system. 6 Each TLR so far recognized binds with specific ligands. For example, TLR-2 predominantly recognizes Gram-positive bacterial components. 6
For some of the diseases that follow, treatment options will be considered only briefly, or not at all. This is because antibiotic sensitivity of some organisms varies widely from country to country, and cost becomes an additional limiting factor to antibiotic choice in some communities. Vaccines are currently available for several bacterial diseases including anthrax, Haemophilus influenzae, and Neisseria meningitidis. 7
The superficial pyogenic infections of the skin (pyodermas) include impetigo and its variants and ecthyma. They also include the superficial infections of the hair follicles, which are dealt with in Chapter 15 (pp. 402–404). In addition, the staphylococcal ‘scalded skin’ syndrome can be included in this category, although the lesions result from the action of bacterial toxin rather than local infection itself.
Impetigo is an acute superficial pyoderma which heals without scar formation. It is the most common bacterial infection of the skin in childhood.8.9.10. and 11. Its incidence appears to be increasing. 12 Adults are sometimes affected, particularly athletes, military personnel, and those in institutions. 13 Minor trauma, especially from insect bites, as well as poor hygiene and a warm, humid climate, all predispose to this infection.14. and 15. Seasonal variation in its occurrence has been noted.16. and 17.
There are two clinical forms of impetigo: a common, vesiculopustular type, and a bullous variant, which is considerably less frequent.18. and 19. Recent studies have shown that Staphylococcus aureus is now the most common organism isolated from the non-bullous type of impetigo,20.21. and 22. which in the past was caused mostly by a group A β-hemolytic streptococcus, sometimes with Staph. aureus as a secondary invader.10. and 13. Anaerobes are now isolated in a number of cases. 22 The bullous form has always been related exclusively to Staph. aureus, usually of phage group II. 23 Both bullous and non-bullous forms of impetigo are associated with exfoliative toxins. Exfoliative toxin genes were present in one study in 100% of Staph. aureus isolates from bullous impetigo and from 57% of isolates from non-bullous impetigo. 5
The incidence of strains of Staph. aureus resistant to the antimicrobial agents used in the treatment of impetigo has been increasing; MRSA accounts for less than 20% of such infections. 24 An ointment containing an equal blend of gentamicin and fusidic acid has been widely used in Japan and some European countries to treat localized impetigo. 24 Sensitivity to fusidic acid has decreased significantly in recent years. 17 Bacitracin is commonly used, but its efficacy is questionable. 25 Topical mupirocin can be an effective treatment option, as are older treatments such as topical gentian violet and vioform. 25 Other topical treatments with some benefit include topical hydrogen peroxide cream, and tea lotion and cream. 25 Topical retapamulin, which belongs to a new class of antibiotics, is an effective and safe treatment for impetigo. 26 Clindamycin has shown excellent activity against most isolates of Staph. aureus and can be used for more serious disease. Other antimicrobials that can be used include dicloxicillin, cloxacillin, cephalexin, or amoxicillin combined with potassium clavulanate. 25
Common impetigo (‘school sores’) commences as thin-walled vesicles or pustules on an erythematous base: the lesions rapidly rupture to form a thick, golden crust. 27 Common impetigo occurs as a solitary lesion or a cluster of several lesions, which may coalesce. It is found on the face or extremities. 21 Local lymphadenopathy may be present.
Some authors use the term impetigo contagiosa (non-bullous impetigo) for this group, and restrict the term common impetigo to a secondary impetigo that may complicate systemic disease, or dermatological conditions that cause a break in the skin. 25
Bullous impetigo is composed of shallow erosions and flaccid bullae, 0.5–3 cm in diameter, with an erythematous rim. 13 The bulla has a thin roof which soon ruptures, resulting in a thin crust. 23 There may be a localized collection of a few bullae, or more generalized lesions.28. and 29. Bullous impetigo is included with the staphylococcal epidermolytic toxin syndrome, as the lesions result from the production in situ of an epidermolytic toxin by staphylococci. 13
Common impetigo is rarely biopsied, as the diagnosis can be made on clinical grounds. An early lesion will show a subcorneal collection of neutrophils, with exocytosis of these cells through the underlying epidermis. A few acantholytic cells are sometimes seen, but this is never a prominent feature. Established lesions show a thick surface crust composed of serum, neutrophils in various stages of breakdown, and some parakeratotic material. Gram-positive cocci can usually be found without difficulty in the surface crust.
In bullous impetigo the subcorneal bulla contains a few acantholytic cells, a small number of neutrophils, and some Gram-positive cocci (Fig. 23.1). 23 In contrast to the lesions of the staphylococcal ‘scalded skin’ syndrome, there is usually a mild to moderate mixed inflammatory cell infiltrate in the underlying papillary dermis. 23
Bullous impetigo. There is a subcorneal blister containing inflammatory cells and degenerate keratinocytes. Gram-positive cocci were found. (H & E)
The staphylococcal ‘scalded skin’ syndrome results from the production of an epidermolytic toxin by certain strains of Staph. aureus, most notably type 71 of phage group II.13. and 30. Our understanding of these bacterial strains is now much more sophisticated. Exfoliative toxins result from the presence of certain genes (eta, etb, and etd) in the organism. These genes encode for ETA, ETB, and ETD respectively. 5 These organisms are responsible for a preceding upper respiratory tract infection, conjunctivitis, or carrier state. Rarely, the syndrome follows a staphylococcal infection complicating varicella or measles.31. and 32.
The SSSS predominantly affects healthy infants and children younger than 6 years, apparently reflecting an inability to handle and excrete the toxin. 33 Rarely, neonates are involved, a condition known in the past as Ritter’s disease (Ritter von Rittershain’s disease). 34 A few cases have been reported in adults, in whom there has usually been underlying immunosuppression (including the acquired immunodeficiency syndrome)35. and 36. and/or renal insufficiency.37.38. and 39. It occurs rarely in healthy adults.40.41.42.43. and 44.
There is a sudden onset of skin tenderness and a scarlatiniform eruption which is followed by the development of large, easily ruptured, flaccid bullae and a positive Nikolsky sign. 34 Desquamation of large areas of the skin occurs in sheets and ribbons. 37 Occasionally, only the scarlatiniform eruption develops. The usual sites of involvement are the face, neck, and trunk, including the axillae and groins. Mucous membranes are not involved.
The disease has a good prognosis in children, with spontaneous healing after several days as a consequence of the formation of neutralizing antibodies to the epidermolytic toxin. 45 In adults a staphylococcal septicemia may ensue and is sometimes fatal. Concurrent SSSS and toxic shock syndrome are extremely rare. 46 A chronic case, evolving over 2 years, has been reported in an adult female patient. 47
Desquamation results from the effects of an exotoxin of low molecular weight (exfoliatin), produced by certain strains of Staph. aureus. There are two forms of exfoliatin recognized – exfoliative toxin A (ETA), which is chromosomally encoded, and exfoliative toxin B (ETB), which is plasmid encoded. 48 Other exfoliative toxins have been described (ETD). 5 Exfoliative toxins act as serine proteases which cleave desmoglein 1 in the superficial epidermis. 49 The condition can be reproduced in newborn mice by the subcutaneous or intraperitoneal injection of these organisms. 50
The treatment of SSSS involves parenteral antibiotics, such as dicloxacillin, to eradicate the Staph. aureus, and appropriate nursing care to prevent the secondary effects of disrupted skin barrier function. 36
The SSSS, which was historically considered (incorrectly) to be a variant of toxic epidermal necrolysis (see p. 53), has been regarded as belonging to the staphylococcal epidermolytic toxin syndrome.51.52. and 53. Also included in this concept are localized and generalized bullous impetigo, which result from the local production (as opposed to production at a distant site, as in SSSS) of a similar staphylococcal epidermolytic toxin. 31 Consequently, in bullous impetigo the organisms may be demonstrated within the lesion. Impetigo is discussed in further detail above.
In the SSSS there is subcorneal splitting of the epidermis (Fig. 23.2). A few acantholytic cells and sparse neutrophils may be present within the blister, although often it is difficult to obtain an intact lesion. A sparse, mixed inflammatory cell infiltrate is present in the underlying dermis. This is in contrast to generalized bullous impetigo, and even pemphigus foliaceus, in which the dermal infiltrate is heavier.
The staphylococcal scalded skin syndrome with subcorneal splitting. (H & E)
Immunofluorescence is negative, in contrast to pemphigus foliaceus, in which intercellular immunoreactants are usually demonstrable.
The staphylococcal toxic shock syndrome was first recognized over a decade ago in healthy menstruating women who used tampons.55. and 56. It results from a toxin produced by certain strains of Staph. aureus that proliferate in the vagina and cervix. The organisms possess the gene tst (toxic shock syndrome toxin gene). 5 The syndrome may also result from the production of one of the staphylococcal enterotoxins. 57 These toxins appear to activate T lymphocytes with the production of various cytokines. 58 The toxic shock syndrome can also complicate wound infections with Staph. aureus. 59 Currently, the incidence of non-menstrual disease exceeds that related to the female genital tract.30. and 60. The clinical features of this syndrome include a fever, hypotension, inflammation of mucous membranes, vomiting and diarrhea, and cutaneous lesions that resemble viral exanthemas or erythema multiforme.61. and 62. The skin lesions undergo desquamation in time.
A streptococcal toxic shock syndrome has also been reported (see below).
The characteristic features of the toxic shock syndrome are small foci of epidermal spongiosis containing a few neutrophils, scattered degenerate keratinocytes, sometimes arranged in clusters, and a superficial perivascular and interstitial cell infiltrate. 61 The infiltrate contains lymphocytes, neutrophils, and sometimes eosinophils. 61 Inflammatory cells often extend into the walls of the superficial dermal vessels, as seen in vasculitis, but there is no fibrin extravasation. Less constant features include irregular epidermal acanthosis, edema of the papillary dermis, extravasation of erythrocytes, and nuclear ‘dust’ in the vicinity of the blood vessels. 61 Focal parakeratosis, containing neutrophils and serum, may also be present.
The streptococcal toxic shock syndrome is caused by virulent strains of exotoxin-producing streptococci, almost always group A organisms such as Streptococcus pyogenes. 63 Recently, a few cases due to group B streptococci have been reported. 64 It often occurs in the setting of deep soft tissue infections, when the portal of entry of the organism appears to be through the skin, but it may complicate burns, surgical wounds, or childbirth. Accordingly it can be seen in several clinical situations such as the young, the immunocompromised, the elderly, and diabetics. Rarely, it has developed in patients taking non-steroidal anti-inflammatory drugs. 30
Clinically there is fever, pain at the site of the deep tissue infection, and skin necrosis and bullae. Renal failure and disseminated intravascular coagulation may occur. 65 A scarlatiniform rash may be present. A streptococcal bacteremia is present in 60% of cases, in contrast to the negative blood cultures in the staphylococcal toxic shock syndrome. 56 The mortality rate is usually as high as 30%; 64 it was 62% in a Chinese outbreak in 2005. 65
Prompt treatment with antibiotics should be undertaken. High dose penicillin G is usually effective, but antibiotics with a broader spectrum are usually commenced first, as the diagnosis may be in doubt.
The changes resemble those in ecthyma gangrenosum (see below). The deep soft tissue lesions, if present, are those of necrotizing fasciitis (see p. 552).
Perianal streptococcal dermatitis, caused by group A β-hemolytic streptococci, has been described almost exclusively in children, although a few adult cases have been reported.66.67. and 68. It presents as perianal erythema with a clearly defined border followed by a desquamating scale and subsequent healing. Systemic symptoms, such as fever, are uncommon69 in contrast to toxin-mediated perineal erythema which occurs abruptly after a bacterial pharyngitis due to Staph. aureus or Streptococcus pyogenes.30. and 70. It can occur not only in young adults but also in childhood. 71
Ecthyma is a deeper pyoderma than impetigo and much less frequent. 18 It has a predilection for the extremities of children, often at sites of minor trauma, which allow entry of the causative bacteria. Group A streptococci, particularly Streptococcus pyogenes, are usually implicated, although coagulase-positive staphylococci are sometimes isolated as well.19. and 72. The lesions, which are sometimes multiple, consist of a dark crust adherent to a shallow ulcer and surrounded by a rim of erythema. Scarring usually results when the lesions heal. 19
Ecthyma gangrenosum is a severe variant of ecthyma seen in 5% or more of immunosuppressed individuals who develop a septicemia with Pseudomonas aeruginosa.73.74.75.76.77. and 78. A septicemia is not invariable. 79 It has also been seen in a harlequin baby, 80 and in previously healthy individuals. 81 It commences as an erythematous macule on the trunk or limbs: the lesion rapidly becomes vesicular, then pustular, and finally develops into a gangrenous ulcer with a dark eschar and an erythematous halo.82.83.84. and 85. Annular lesions are rare. 86 Periocular lesions have been reported in a diabetic patient. 87 Constitutional symptoms are usually present. Patients with solitary lesions have a better prognosis than those with multiple lesions. Similar necrotic ulcers have been reported in association with Aspergillus, Candida, 88 and Exserohilum89 infection, Morganella morganii, 90Citrobacter freundii, 91Chromobacterium violaceum, 92 candidosis, and following pseudomonas folliculitis (but usually without septicemia), 73 and cutaneous infections treated with antibiotics. 93Pseudomonas aeruginosa septicemia may result in the development of pustules, 94 bullae, 95 intertrigo, 96 or of a nodular cellulitis97 rather than ecthyma gangrenosum. 98 It needs to be distinguished from purpura fulminans in which disseminated intravascular coagulation (DIC) accompanies the infection (see p. 199).99.100. and 101.
In ecthyma there is ulceration of the skin with an inflammatory crust on the surface. There is a heavy infiltrate of neutrophils in the reticular dermis, which forms the base of the ulcer. Gram-positive cocci may be seen within the inflammatory crust.
Ecthyma gangrenosum shows necrosis of the epidermis and the upper dermis, with some hemorrhage into the dermis. 82 The epidermis may separate from the dermis. A mixed inflammatory-cell infiltrate surrounds the infarcted region. In some cases there is a paucity of inflammation.82. and 83. A necrotizing vasculitis with vascular thrombosis is present in the margins. 97 Numerous Gram-negative bacteria are usually present between the collagen bundles, and sometimes in the media and adventitia of small blood vessels.
Cellulitis is a diffuse inflammation of the connective tissue of the skin and/or the deeper soft tissues.19.102. and 103. It is therefore a deeper pyoderma than impetigo and some cases of ecthyma, although ecthyma gangrenosum could be included in this category. Clinically, cellulitis presents as an expanding area of erythema, which is usually edematous and tender. 104 Necrosis and hemorrhage sometimes supervene.105.106.107. and 108. In the past, these infections were usually caused by β-hemolytic streptococci and/or coagulase-positive staphylococci.19. and 109. A diverse range of organisms is now implicated in the causation of cellulitis.102.110.111.112.113.114.115.116.117.118. and 119. The injection of ricin in a suicide attempt resulted in a septic and toxic cellulitis in one patient. 120 Neutropenic and leukemic patients are now being seen with erythematous nodules on the leg caused by the opportunistic pathogen Stenotrophomonas (Xanthomonas) maltophilia.121.122. and 123. This organism has also resulted in digital necrosis in an immunocompetent farmer. 124 Pyogenic sporotrichoid lymphangitis is a rare presentation. 125 Lower extremity cellulitis appears to be increased in patients who have undergone saphenous venectomy for coronary artery bypass graft surgery,126. and 127. but it is also seen in chronic venous insufficiency, and obesity. 128 Gangrenous cheilitis has been reported in a child with myeloperoxidase deficiency. 129 Facial cellulitis may occur secondary to a dental abscess. 130
Many different clinical variants of cellulitis have been reported, some with overlapping clinical features and causative bacteria. 104 This has led to a proliferation of terms for these different variants. The term ‘gangrenous and crepitant cellulitis’ has been used for a subset with prominent skin necrosis and/or the discernible presence of gas in the tissues.105. and 131. The term ‘hemorrhagic cellulitis’ has also been used for this group, which includes progressive bacterial synergistic gangrene. It has been suggested that TNF-α is responsible for the damage to keratinocytes and vascular endothelium. 132 Subcutaneous and/or dermal abscesses are another manifestation of deep infection by various bacteria.133.134.135.136.137.138.139.140.141. and 142.
The cellulitides are characterized histopathologically by an infiltrate of neutrophils throughout the dermis and/or the subcutaneous tissue, with variable subepidermal edema and vascular ectasia. In those variants with necrosis there is usually a necrotizing vasculitis, which may be associated with fibrin thrombi in the lumen.143. and 144. Bacteria are often numerous in the group with necrosis, although usually only a few can be isolated in the other variants. 145
Treatment will usually depend on the antibiotic sensitivity of the causative organism. An increasing problem, of particular interest to dermatologists, is the emergence of community-acquired, methicillin-resistant Staph. aureus, which can be highly virulent.146.147.148. and 149. This form of infection is now an increasingly common condition among athletes. 150 It usually presents as skin and soft-tissue infections, especially abscesses. 151 Many such abscesses respond to drainage alone, but some will require antibiotic therapy. Trimethoprim-sulfamethoxazole is an inexpensive and effective choice for many such patients. 151 It may be used with rifampin. 152 Resistance is emerging to the fluoroquinolones. 151
Erysipelas is a distinctive type of cellulitis which has an elevated border and spreads rapidly.19.153. and 154. It is more common in males, and most prevalent in patients 65 years and older. 155 Whereas the incidence of bacterial cellulitis of the leg in the United States was reported to be 6.62 per 1000 in 2004, erysipelas was much less common (0.14 per 1000). 156
Vesiculation may develop, particularly at the edge of the lesion. An uncommon bullous variant, usually confined to the lower legs, has been described. 157 The condition occurs particularly on the lower extremities, and less commonly on the face.158. and 159. Underlying diabetes mellitus, peripheral vascular disease or lymphedema may be present. 158 Erysipelas may complicate the upper limb lymphedema that follows the treatment of breast cancer. 160 The causative group A streptococci,103. and 161. or other organisms,162. and 163. gain entry through superficial abrasions. It is associated with pain, swelling, and fever. Bacteremia is common. 19 Osteoarticular complications are not uncommon. 164
An erysipelas-like erythema, usually on the lower legs, is seen in familial Mediterranean fever, an autosomal recessive disease affecting certain ethnic groups. 165 The histopathology has features more in keeping with a neutrophilic dermatosis than erysipelas.
The term pseudoerysipelas has been given to a recurrent hemolysis-associated erythematous eruption of the lower legs in a patient with hereditary spherocytosis. 166 No infection was present. 166
Treatment of erysipelas is usually with β-lactam antibiotics but macrolides are the second choice. 155 Benzathine penicillin G, intramuscularly at 14-day intervals, has been used as prophylactic antibiotherapy in patients who have experienced recurrent erysipelas in lymphedematous upper limbs following the treatment of breast cancer. 160 Long-standing treatment for lymphedema of the lower limbs is essential in order to prevent recurrence of erysipelas and aggravation of the pre-existing lymphatic impairment. 167
There is marked subepidermal edema, which may lead to the formation of vesiculobullous lesions. Beneath this zone there is a diffuse and usually heavy infiltrate of neutrophils, but abscesses do not form. The infiltrate is sometimes accentuated around blood vessels. There is often vascular and lymphatic dilatation. In healing lesions the dermal infiltrate diminishes, and granulation tissue may form immediately below the zone of subepidermal edema (Fig. 23.3). Direct immunofluorescence has been used to confirm the streptococcal etiology of most cases of erysipelas. 161
Erysipelas. (A) Acute lesion with marked subepidermal edema. (B) This healing lesion shows little inflammation in the upper dermis. (H & E)
Erysipeloid is an uncommon infection, usually found on the hands, which clinically resembles erysipelas. 168 The causative organism, Erysipelothrix rhusiopathiae, is a contaminant of dead organic matter, and infection with this organism is an occupational hazard for fish and meat handlers. 169 The incubation period varies from 1 to 7 days after inoculation. 170 Less commonly, multiple cutaneous lesions or systemic spread of the organism may occur. Erysipeloid-like eruptions have been reported in several patients receiving chemotherapy with gemcitabine. 171
Treatment of erysipeloid with penicillin for 7–10 days results in dramatic improvement.169. and 170.
There is usually massive edema of the papillary dermis overlying a diffuse and polymorphous infiltrate composed of lymphocytes, plasma cells, and variable numbers of neutrophils. Sometimes there is spongiosis of the epidermis, leading to intraepidermal vesiculation. Organisms are not demonstrable in tissue sections, even with a Gram stain, possibly because they are present in the L form (without a cell wall). 168
Blistering distal dactylitis is an uncommon yet distinctive infection localized to the volar fat pad of the distal phalanx of the fingers.19.172.173.174. and 175. Group A streptococci are usually implicated, although rarely other organisms have been isolated.176. and 177. The blistering results from massive subepidermal edema.
In addition to its use as a synonym for deep pyogenic infection (see above), the term ‘cellulitis’ is sometimes used in a more restricted sense for spreading inflammation of the cheek,178.179. and 180. periorbital area181. and 182. or the perianal region,174.183.184.185. and 186. or in the margins of wounds, 19 or injecting sites. 187 The lesions lack the distinct border of erysipelas. 19 Various organisms have been implicated as the cause of this condition, 188 including Staph. aureus in patients with HIV infection, 189Haemophilus influenzae type b in the case of facial lesions,179. and 190. and Vibrio vulnificus in some infections of the extremities.191.192.193.194.195.196.197. and 198. The latter organism can produce various lesions, including septicemia, hemorrhagic bullae,199. and 200. cellulitis, and necrotizing fasciitis. 201Pasteurella multocida has been implicated in the wound infection and cellulitis that may follow animal bites. 202Vibrio cholerae (non-01 type) can rarely produce a cellulitis or infect a pre-existing wound.203. and 204. Escherichia coli has also been implicated, particularly in immunocompromised individuals. 205 Varicella infection is complicated, rarely, by a cellulitis resulting from group A β-hemolytic streptococci or Staph. aureus. 206
Necrotizing fasciitis is a rare and distinct form of cellulitis that rapidly progresses to necrosis of the skin and underlying tissues.207.208.209.210.211.212.213.214. and 215. It occurs mostly in adults but cases in children have been reported. 216 The term ‘flesh-eating bacteria’ has appeared in the media, highlighting the progressive necrotizing nature of the disease. 217 It involves tissues at a deeper level than erysipelas, and may spread into the underlying muscle. 218 Necrotizing fasciitis commences as a poorly defined area of erythema, usually on the leg, 207 or perineal region. 219 Bilateral periorbital involvement has been recorded. 220 Serosanguineous blisters develop and subsequently necrosis occurs at their center.221. and 222. It often follows a penetrating injury. Uncommonly, the condition follows surgery; 223 in one case it followed mosquito bites. 224 There may be underlying diabetes, alcoholism, or some other immune system deficiency.225. and 226. There is a strong association with the use of non-steroidal anti-inflammatory drugs (NSAIDs). 227 It may occur in intravenous drug users.228. and 229. Constitutional symptoms may be present, and there is a significant mortality. 218 The term ‘streptococcal toxic shock syndrome’ has been applied to this systemic illness, 230 although it more properly refers to a discrete entity with some similarity to the toxic shock syndrome of staphylococcal origin (see p. 550). 30 Various organisms have been isolated,231.232. and 233. particularly group A streptococci.222.234.235. and 236. Often the infection is polymicrobial.216. and 237. A zygomycete has also been isolated from this condition. 238 Rapid diagnosis kits are available to confirm cases of streptococcal origin. Protein S deficiency may be responsible for the necrosis in some cases. 239
Immediate treatment of necrotizing fasciitis with penicillin, if group A streptococci are involved, or with clindamycin plus cefoperazone sodium if the organism is not known, is recommended once a diagnosis is made, together with surgical debridement. 216 The antibiotics should then be tailored to the sensitivity of any organism(s) cultured. 235
Fournier’s gangrene of the scrotum is a closely related entity.221. and 240. It is usually found in elderly men, with an underlying disease such as diabetes. Cases have been reported in younger persons. 241 It is a rare complication of the use of all-trans-retinoic acid as induction therapy in the treatment of acute promyelocytic leukemia. Its response to corticosteroids suggests that Fournier’s gangrene is a localized vasculitis and represents a local Schwartzmann phenomenon. 242 Enterobacteria are common isolates, although a mixed growth is often seen.243. and 244. In one recent series the mortality was nearly 10%. 243 Treatment of Fournier’s gangrene includes fluid resuscitation, empirical broad-spectrum antibiotics until the antibiotic sensitivities of the organism(s) are known, and surgical debridement of necrotic tissue. 240
Necrotizing fasciitis is a form of septic vasculitis with inflammation of the walls of vessels, sometimes associated with occlusion of the lumen by thrombi. 144 There is a mixed inflammatory cell infiltrate in the viable tissues bordering the areas of necrosis. The necrosis involves the epidermis, dermis, and upper subcutis.
There are several rare but distinct clinicopathological entities that belong to the category of deep pyogenic infections. They include clostridial myonecrosis (gas gangrene), progressive bacterial synergistic gangrene, and erosive pustular dermatosis of the scalp, legs, and other sites. They will be discussed in turn.
Clostridial myonecrosis (gas gangrene) is associated with muscle and soft tissue necrosis. Cutaneous lesions, including bullae and necrosis, may overlie the deeper lesions. The usual causative organisms in gas gangrene are clostridial species; non-clostridial cases are infrequently reported. 245 Wound botulism has been reported in black tar heroin users. 246 Large Gram-positive bacilli are usually present in the affected tissues. 105 A bacterium related to the genus Clostridium – Bacillus piloformis – has produced localized verrucous lesions in a patient infected with HIV-1. 247Bacillus cereus infection has been associated with a single necrotic bulla in a patient with a lymphoma. 248
Treatment includes the immediate surgical debridement of damaged tissue and the use of antibiotics such as penicillin in high doses. Hyperbaric oxygen therapy is also effective but it should not delay the other measures already mentioned.
Progressive bacterial synergistic gangrene is characterized by indurated ulcerated areas with a gangrenous margin, usually developing in operative wounds.105. and 249. This condition, also known as Meleney’s ulcer, is often associated with a mixed growth of peptostreptococci and Staph. aureus or enterobacteriaceae. 105
Erosive pustular dermatosis is an uncommon disorder of the elderly, involving predominantly the sun-damaged scalp. It presents with widespread erosions and crusted sterile pustules, leading to scarring alopecia.250.251. and 252. Intact pustules are rarely present, despite the name. 253 A clinically similar rash has been reported on the legs, particularly in a background of chronic venous stasis.253.254. and 255. Lesions on the face and other body sites have also been reported, and it has been suggested that chronic, non-healing, shallow erosions on actinically damaged, or otherwise atrophic skin are related conditions. The condition has a female predominance and most affected individuals are over 65 years of age; it has also been reported in a 15-year-old boy, 253 and in infants. 256
Its exact nosological position is uncertain, but there are some morphological similarities to blastomycosis-like pyoderma (see below). Trauma, previous herpes zoster infection, recent cryotherapy, 5-fluorouracil application, radiation therapy, and surgery have all been implicated as predisposing factors.253.257.258.259.260.261. and 262. Cases in infants may follow perinatal scalp injury. 256Staph. aureus is sometimes isolated, but these organisms may be secondary invaders. 250Amicrobial pustulosis associated with autoimmune diseases is probably part of the spectrum. It may involve the scalp as well as the flexures. It has responded to zinc therapy. 263
Treatment with potent topical corticosteroids is frequently used. Topical tacrolimus ointment and calcipotriol (calcipotriene) cream can also be used. Oral zinc sulfate and isotretinoin have been tried in some cases.264.265. and 266. The lesions do not respond to topical or oral antibiotics. 256
The skin is usually ulcerated, but intact atrophic epidermis may cover other involved areas. In such cases there is exocytosis of inflammatory cells. There is variable crusting overlying ulcerated skin and atrophic areas. Intact pustules are rarely seen. The dermal infiltrate is moderate to marked, and consists of lymphocytes and variable numbers of plasma cells. Scarring is progressive. There may be a few foreign body giant cells in areas of follicular destruction. 264
Blastomycosis-like pyoderma is an unusual form of pyoderma that presents with large verrucous plaques studded with multiple pustules and draining sinuses.267. and 268. There may be an underlying disturbance of immunological function in some cases.268. and 269. A variant of this condition is found in subtropical areas of Australia in the actinically damaged skin of the elderly, particularly on the forearm.270. and 271. This has been known as ‘coral reef granuloma’, on the basis of its clinical appearance. 272 Actinic comedonal plaque, in which plaques and nodules with a cribriform appearance develop in sun-damaged skin, 273 appears to be the end-stage of a similar but milder inflammatory process.270. and 271. Similar lesions have been reported at the margins of tattoos.270. and 274. Actinic comedonal plaque has also been regarded as an ectopic form of the Favre–Racouchot syndrome (see p. 342). 275
Bacteria, particularly Staph. aureus and species of Pseudomonas and Proteus, have been isolated from biopsies.268.270.276. and 277. Sun-damaged skin is known to diminish local immune responses, and this factor is probably important in the variant found in Australia.278. and 279.
Response to antibiotics such as oral doxycycline is often poor, despite in-vitro susceptibility of the organism to tetracyclines. 280 Minocycline has also been used. Local ablative measures such as curettage and cautery, carbon dioxide laser, or cryotherapy may be used if surgical excision is unsuitable. 281 Low-dose oral acitretin for 3–4 months has been used successfully. 280
There is a heavy inflammatory infiltrate throughout the dermis, with multiple small abscesses set in a background of chronic inflammation (Fig. 23.4). 268 A few granulomas are occasionally present, but these are usually related to elastotic fibers. There is prominent pseudoepitheliomatous hyperplasia, which in some areas appears to result from hypertrophy of the follicular infundibulum. Intraepidermal microabscesses are present, and these probably represent the attempted transepidermal elimination of the dermal inflammatory process. Solar elastotic fibers are present in the variants known as ‘coral reef granuloma’ and ‘actinic comedonal plaque’. 273 There may be some dermal fibrosis in healing lesions, although actinically damaged fibers usually persist in the upper dermis.
Blastomycosis-like pyoderma. (A) There is dermal suppuration adjacent to enlarged follicular structures which become draining sinuses. (B) Suppurative granulomas are often present. (H & E)
The corynebacteria are a diverse group of Gram-positive bacilli which include Corynebacterium diphtheriae, the causative organism of diphtheria, as well as a bewildering number of species that are found on the skin as part of the normal flora and which defy classification. 282 These latter organisms are usually referred to as diphtheroids or coryneforms. Certain strains have the ability to produce malodor of the axilla. 283 Three skin conditions appear to be related to an overabundance of these coryneforms: erythrasma, trichomycosis, and pitted keratolysis. 282 Interestingly, the three have been reported to coexist in some individuals.284. and 285. Rarely, other species of corynebacteria have been incriminated as a source of infection in diabetics286 or immunocompromised patients, the most important being the JK group (C. jeikeium).287. and 288. These organisms have been found in patients with heart prostheses and endocarditis, and recently as a cause of cutaneous lesions in immunocompromised patients. 287 They may produce a histological picture mimicking botryomycosis (see p. 602). 288
Topical sodium fucidate has been used to treat cutaneous disease. 285 Oral erythromycin can be used for more extensive infections. 285
Cutaneous diphtheria is a rarely diagnosed entity which is still found very occasionally in tropical areas.289.290. and 291. The typical lesion is an ulcer with a well-defined irregular margin; the base is covered with a gray slough. Systemic effects, from the absorption of exotoxin, and severe lymphadenitis may develop. Cutaneous carriers also occur.
The treatment of diphtheria requires the administration of a specific antitoxin as soon as the diagnosis is made, and the use of penicillin or erythromycin.
There is necrosis of the epidermis and varying depths of the dermis. The base of the ulcer is composed of necrotic debris, fibrin, and a mixed inflammatory infiltrate. The bacilli are often difficult to see in tissue sections.
Erythrasma, caused by a diphtheroid bacillus Corynebacterium minutissimum, presents as well-defined, red to brown finely scaling patches with a predilection for skin folds, particularly the inner aspect of the thigh just below the crural fold.292.293. and 294. A rare generalized disciform variant has been reported. 294 This organism has been cultured from granulomatous nodules on the leg in an HIV-infected man. 295 Erythrasma is a not uncommon asymptomatic infection in the obese, and in diabetics and patients in institutions, particularly in humid climates.292. and 293. Examination of affected skin under a Wood’s ultraviolet lamp shows a characteristic coral-red fluorescence (because of the presence of porphyrins).
The treatment of erythrasma involves the topical application of one of the imidazole antifungal agents such as clotrimazole, ketoconazole, or miconazole. Whitfield’s ointment has been used for interdigital lesions. Erythromycin can be used for extensive or recurrent cases.
The biopsy often appears normal when hematoxylin and eosin preparations are examined, erythrasma being an example of a so-called ‘invisible dermatosis’. Small coccobacilli can be seen in the superficial part of the stratum corneum in Gram preparations. They are also seen in PAS (periodic acid–Schiff) and methenamine silver preparations (Fig. 23.5).
Erythrasma. Minute organisms can be seen in the stratum corneum. There is no inflammatory response. (Periodic acid–Schiff)
Electron microscopy has confirmed the bacterial nature of erythrasma. It has also shown decreased electron density in keratinized cells, with dissolution of normal keratin fibrils at sites of proliferation of organisms.298. and 299.
Trichomycosis is a bacterial infection of axillary hair (trichomycosis axillaris) and, uncommonly, pubic hair (trichomycosis pubis).300. and 301. There are usually pale yellow concretions attached to the hair shaft: these are large bacterial colonies. Sometimes the casts are red, and rarely they are black. 301 They may be the source of an offensive odor. The causative organism was originally designated as Corynebacterium tenuis, but it now seems that at least three species are involved.301. and 302. The infecting bacteria are generally believed to produce a cement-like substance which they use to adhere to the hair shaft and to form the large concretions; 301 this traditional view has been challenged, 303 and it has now been suggested that the sheath substance in which the organisms are embedded is apocrine sweat. 303 Sometimes the bacteria invade the superficial hair cortex. 304 Trichomycosis must be distinguished from other extraneous substances attached to the hair shaft.
The use of an antiperspirant will usually control the disease. If there is a heavy growth of organisms on the hairs, clipping followed by the application of a topical antibacterial agent will hasten resolution.
Pitted keratolysis is characterized by multiple asymptomatic pits and superficial erosions on the plantar surface of the feet, particularly in pressure areas.305.306.307.308.309. and 310. Rarely, the palms are involved.306.311. and 312. It occurs predominantly in young adults; there is a male predominance. 313 Sometimes there is brownish discoloration of involved areas, giving them a dirt-impregnated appearance. 314 Pitted keratolysis is more common if the feet are moist from hyperhidrosis or because the climate is hot. 315 It is often associated with the wearing of occlusive and protective shoes. 313 Malodor is common. 310 The pits are thought to result from the keratolytic activity of species of Corynebacterium, but Dermatophilus congolensis307. and 316. and Micrococcus sedentarius317 have also been incriminated.
As with erythrasma, the topical application of one of the imidazole antifungal agents is usually effective. Fucidin ointment has also been used. Any excess sweating should be appropriately controlled.
The pits appear as multiple crateriform defects in the stratum corneum. In early lesions there are areas of pallor within the stratum corneum (Fig. 23.6). In the base and margins of the pits there are fine filamentous and coccoid organisms that are Gram-positive and argyrophilic with the methenamine silver stain. It has been suggested that two types of pitted keratolysis can be distinguished histologically. 318 In the superficial or minor type there is only a small depression due to focal lysis. Coccoid bacteria are found on the surface of the stratum corneum. In the classical or major type the organisms exhibit dimorphism with septate ‘hyphae’ as well as coccoid forms, which extend into the stratum corneum forming more definite pits. 318
Pitted keratolysis. (A) The stratum corneum adjacent to an area of pitting shows zones of pallor corresponding to foci containing the causative organisms. (H & E) (B) They can be seen in the pale areas of the stratum corneum using silver stains. (Silver methenamine)
Primary infections of the skin by Neisseria meningitidis and N. gonorrhoeae are rare because these organisms are unable to penetrate intact epidermis. However, cutaneous lesions do occur quite commonly in meningococcal and gonococcal septicemia; they take the form of a vasculitis (see Ch. 8, p. 213).
Cutaneous lesions, which may take the form of erythematous macules, nodules, petechiae, or small pustules, occur in 80% or more of acute meningococcal infections (see p. 213). Features of disseminated intravascular coagulation are invariably present. Chronic meningococcal septicemia is comparatively rare; it is characterized by the triad of intermittent fever, arthralgia, and vesiculopustular or hemorrhagic lesions of the skin. The hemorrhagic lesions are usually a manifestation of purpura fulminans, which develops in 15–25% of those with meningococcemia. 320 It is a predictor of poor outcome. 320 Chronic meningococcemia is a rare complication in patients with the acquired immunodeficiency syndrome. 321 A N. meningitidis-specific PCR assay is available for testing skin biopsy specimens in suspected chronic meningococcemia. 322
The cutaneous lesions in meningococcal septicemia show an acute vasculitis, with fibrin thrombi in the small blood vessels of the dermis and extravasation of fibrin. There are neutrophils in and around the vessels, but the infiltrate is not as heavy as it is in hypersensitivity vasculitis. Leukocytoclasis is not usually a conspicuous feature.
In pustular lesions of chronic meningococcemia there are intraepidermal and subepidermal collections of neutrophils. A vasculitis is present in the dermis; the infiltrate contains some lymphocytes in addition to neutrophils.
Urethritis (gonorrhea) is the usual manifestation of infection with Neisseria gonorrhoeae. There is now a high incidence of this disease among HIV-infected men who have sex with men. 323 This sexually transmitted disease may also infect the accessory glands of the vulva and the median raphe of the penis. 324 Primary infections of extragenital skin are very rare, although pustular lesions on the digits have been reported. 325 Cases of non-gonococcal urethritis due to species of Mycoplasma are not uncommon. The macrolide antibiotics appear to be more effective than tetracyclines in this group of infections. 326
Gonococcal septicemia, both acute and chronic, may result in a cutaneous vasculitis; the lesions resemble those seen in meningococcal septicemia (see above).327. and 328.
First-line treatment for gonorrhea depends very much on the antibiotic susceptibility of the organism, in a particular region. In parts of Europe, resistance to ciprofloxacin exceeds 50%. 329 Antibiotics such as cefixime, ceftriaxone, spectinomycin, and in some cases azithromycin or ciprofloxacin have been used. 329
Primary pustular lesions on the penis or extragenital skin are usually ulcerated, with a heavy inflammatory cell infiltrate in the underlying dermis. There are numerous neutrophils, often forming small abscesses. Gram-negative intracellular diplococci can usually be found in tissue sections, but they are more easily found in smears made from the purulent exudate on the surface of the lesion.
In gonococcal septicemia the cutaneous lesions resemble those seen in meningococcal septicemia (see above); the appearances are those of a septic vasculitis (see p. 213).
The cutaneous mycobacterioses include leprosy and tuberculosis, as well as a diverse group of infections caused by various environmental (atypical, non-tuberculous) mycobacteria. Within these three categories there are clinicopathological variants which are sometimes given the status of distinct entities; for example, infections by Mycobacterium ulcerans and M. marinum (Buruli ulcer and swimming pool granuloma, respectively) are often considered separately from infections caused by other non-tuberculous (atypical) mycobacteria.
Established mycobacterial infections generally give rise to a granulomatous tissue reaction, although considerable variability exists in the histopathological appearances of individual lesions. 330 These aspects will be considered further below.
Tuberculosis of the skin has been declining in incidence all over the world, although it is still an important infective disorder in India and parts of Africa.331.332.333.334. and 335. The epidemic of HIV/AIDS in parts of Africa has been followed by an epidemic of tuberculosis in its wake. 336 It has been estimated that nearly eight million new cases of tuberculosis occur in the world each year. 337 Cutaneous tuberculosis is a relatively rare manifestation of tuberculosis accounting for only 0.14% of all reported cases. 338 With the eradication of tuberculosis in cattle, human infections with Mycobacterium bovis are rarely seen these days.339.340. and 341. Accordingly, cutaneous tuberculosis can be categorized into two major etiological groups: infections caused by Mycobacterium tuberculosis, and those caused by non-tuberculous (atypical) mycobacteria. Infections by non-tuberculous mycobacteria will be considered separately. In some of the so-called ‘developed countries’, such infections are more numerous than those caused by M. tuberculosis.
Not all individuals exposed to M. tuberculosis become infected. There is a complex interaction between the organism, the environment, and the host. 342 Corticosteroids may upset this balance leading to the reactivation of non-active disease. 343 The increasing use of biological therapies for the treatment of inflammatory skin diseases, particularly psoriasis, has also led to an increase in tuberculosis. 344 Genetic susceptibility to Mycobacterium tuberculosis (OMIM 607948) involves many genes. One such gene maps to chromosome 2q35, which includes the NRAMP1 gene (natural resistance-associated macrophage protein 1), 342 and the MTBS1 gene. Another TB susceptibility locus is found at chromosome 8q12–q13. X-linked susceptibility has also been proposed. Patients with hypomorphic mutations of the nuclear factor κB essential modulator gene may also have increased susceptibility to mycobacterial disease. 345 A review on the genetically determined susceptibility to mycobacterial infection was published in 2008. 346 The review discussed in detail the genetic abnormalities in the interleukin 12 (IL-12) dependent, high output interferon-γ (IFN-γ) pathway that result in increased susceptibility to infection. 346
Infections with M. tuberculosis have traditionally been classified into primary tuberculosis, when there has been no previous exposure to the organism, and secondary tuberculosis, resulting from reinfection with the tubercle bacillus. 331 Reinfection (secondary) tuberculosis of the skin is subdivided on the basis of various clinical features into lupus vulgaris, tuberculosis verrucosa, scrofuloderma, orificial tuberculosis, and disseminated cutaneous tuberculosis. 347 The tuberculids are a further category and were thought to represent a cutaneous reaction to a tuberculous infection elsewhere in the body, there being no detectable bacilli in the tuberculid skin lesions by conventional techniques.
This traditional classification has been disparaged somewhat, and a classification based on the presumed route of infection has been proposed.348. and 349. Several modifications have already been suggested, but its basic format remains as follows:350.351. and 352.
• infections due to inoculation from an exogenous source
• infections from an endogenous source, both contiguous (scrofuloderma in the traditional classification) and from autoinoculation (orificial tuberculosis)
• infections resulting from hematogenous spread.
The last of these three categories can be further subdivided into lupus vulgaris, acute disseminated tuberculosis, and the formation of cutaneous nodules or abscesses.
This relatively new system of classification has the advantage of applying to infections with atypical mycobacteria as well as those caused by M. tuberculosis. However, it still requires assumptions to be made when an attempt is made to classify an individual case. Furthermore, as infections with certain atypical mycobacteria (Buruli ulcer and swimming pool granuloma) are established clinical entities, it seems unlikely that this new classification will offer many advantages over the traditional one. Such has been the case. It should also be emphasized that cases occur which defy classification by any means, and it is quite appropriate to diagnose ‘cutaneous tuberculosis’ in these cases, pending the completion of cultural identification (if obtained).347. and 353. Various automated techniques, using the polymerase chain reaction (PCR), now provide a rapid and specific method for the identification of M. tuberculosis from tissue samples.354.355.356.357.358.359. and 360. However, despite general enthusiasm for this technique,361.362.363.364.365.366.367. and 368. several studies have concluded that PCR was not of much use in paucibacillary forms of cutaneous tuberculosis, particularly using paraffin-embedded tissue.369. and 370. Two commercially available interferon-γ release assays (IGRAs) are now being used in place of tuberculin skin tests for the detection of asymptomatic infections. 371M. tuberculosis has also been identified by PCR in some cases of sarcoidosis. 372
Any classification of cutaneous tuberculosis should also make provision for the complications of BCG vaccination. 373 These include local abscesses and secondary bacterial infections, lupus vulgaris,374.375. and 376. lichen scrofulosorum, 377 lymphadenitis, tuberculids, 378 scrofuloderma-like lesions,379. and 380. and local keloid formation. 381 Granulomatous nodules have developed at the injection site following a long period of latency.382. and 383. Fatalities have been recorded following BCG vaccination of individuals who are immunocompromised and develop disseminated disease.384.385.386.387.388. and 389. There is also a genetic predisposition to disseminated disease following BCG vaccination. It is closely related to the genetic abnormalities associated with atypical mycobacteriosis (see below).
The histological pattern of the BCG test site may be erythema multiforme-like, basal spongiotic in type, or have a non-specific perivascular infiltrate of lymphocytes. If active tuberculosis is present, the test site frequently has the more severe erythema multiforme-type reaction, sometimes with bulla formation. 390
Of interest is the recent use of polyclonal anti-Mycobacterium bovis antibodies to detect bacterial and fungal microorganisms in paraffin-embedded tissue. 391 This technique is particularly useful in cases with only small numbers of organisms.
The following classification will be followed here:
• primary tuberculosis
• lupus vulgaris
• tuberculosis verrucosa
• scrofuloderma
• orificial tuberculosis
• disseminated cutaneous tuberculosis
• tuberculids.
In a series of 153 cases with cutaneous tuberculosis reported from Pakistan, 41.2% had lupus vulgaris, 35.3% scrofuloderma (mainly children), and 19% tuberculosis verrucosa. 392
Primary (inoculation) tuberculosis of the skin is the cutaneous analogue of the pulmonary Ghon focus.393. and 394. One to three weeks after introduction of the organism by way of a penetrating injury, a red indurated papule appears.395.396.397.398.399.400. and 401. This subsequently ulcerates, forming a so-called ‘tuberculoid chancre’. Ulcerated lesions may also be a manifestation of secondary (reinfection) tuberculosis, 402 when the source of infection may be endogenous or from inoculation, although secondary inoculation tuberculosis usually presents as tuberculosis verrucosa (see below). 403 Sporotrichoid lesions have developed following a primary inoculation lesion. 404 Regional lymphadenopathy usually develops in primary tuberculosis.
Primary tuberculosis may be associated with tattooing,395. and 405. ritual circumcision, nose piercing, 406 inoculation of homeopathic solutions, 407 or injury by contaminated objects408 to laboratory workers, surgeons, or to prosectors.393.409. and 410. Sometimes no obvious source of infection can be identified, particularly in children.411. and 412. Atypical (non-tuberculous) mycobacteria are now implicated more often than M. tuberculosis in the etiology of primary tuberculous infection of the skin.
Early lesions show a mixed dermal infiltrate of neutrophils, lymphocytes, and plasma cells. This is followed by superficial necrosis and ulceration. After some weeks tuberculoid granulomas form, and these may be accompanied by caseation necrosis. 347 Acid-fast bacilli are usually easy to find in the early lesions, but there are very few bacilli once granulomas develop.
Lupus vulgaris is the most common form of reinfection tuberculosis, occurring predominantly in young adults.413. and 414. There is one report of its occurrence in siblings. 415 It is caused almost exclusively by M. tuberculosis although rare reports implicating the M. avium-intracellulare complex, M. fortuitum, 416 and M. bovis have been published.339.417.418. and 419. It has followed BCG vaccination,376.420.421. and 422. and also tattoo inoculation. 423 Lupus vulgaris affects primarily the head and neck region, 424 although in southeast Asia it appears to be more common on the extremities and buttocks.425.426.427. and 428. Penile involvement can occur. 429 Lesions involving the nose and face can be destructive. Alopecia often develops in lesions of the scalp. 430 The usual picture is of multiple erythematous papules forming a plaque, which on diascopy shows small ‘apple jelly’ nodules.431. and 432. Crusted ulcers, local cellulitis, 433sporotrichoid lesions,434. and 435. ‘turkey-ear’ lesions, 436 and dry verrucous plaques are sometimes seen. 425 It may develop within a scar, 437 or following tattooing. 438 The disease runs a chronic course and may result in significant scarring. Late complications include the development of contractures, lymphedema, squamous carcinoma330.348.439.440.441. and 442. and, rarely, basal cell carcinoma, 443 malignant melanoma, 444 and cutaneous lymphomas.445. and 446.
In lupus vulgaris there are tuberculoid granulomas with a variable mantle of lymphocytes in the upper and mid dermis. 413 The granulomas have a tendency to confluence (Fig. 23.7). Rarely they have a perifollicular arrangement. 447 Caseation is sometimes present. If prominent caseation is present in a facial lesion, granulomatous rosacea also needs consideration. 448 Multinucleate giant cells are not always numerous. Langerhans cells are present in moderate numbers in the granulomas. 449 The overlying epidermis may be atrophic or hyperplastic, but only rarely is there pseudoepitheliomatous hyperplasia. Transepidermal elimination of granulomas through a hyperplastic epidermis is rarely seen. 450 Bacilli are usually sparse and difficult to demonstrate in sections stained to show acid-fast organisms. 445 They can now be demonstrated using PCR.451. and 452. In one report, Michaelis–Gutmann bodies were present in macrophages in the infiltrate. 453 Variable fibrosis accompanies the lesions. Effective antituberculous therapy leads to a substantial reduction of fibrosis. 454
Lupus vulgaris. There are tuberculoid granulomas, with a tendency to confluence, throughout the dermis. (H & E)
Sometimes the histological appearances resemble sarcoidosis, with only a relatively sparse lymphocytic mantle around the granulomas: a consequent delay in making the correct diagnosis is common in such cases.348.455. and 456.
An atypical CD30+ lymphoid infiltrate has also been described. The infiltrate was eventually regarded as being of reactive type. 457
Tuberculosis verrucosa is an uncommon form of cutaneous tuberculosis resulting from inoculation of organisms into the skin in individuals with good immunity.458.459. and 460. It may occur as an occupational hazard in the autopsy room. A verrucous plaque forms on the back of the hand, elbows, or fingers.347.461.462.463. and 464. The lower extremities are more often involved in cases occurring in India and Hong Kong.331.465.466.467.468.469. and 470.
Scrofuloderma is tuberculous involvement of the skin resulting from direct extension from an underlying tuberculous lesion in lymph nodes or bone.332.471.472.473.474. and 475. The term has also been used, incorrectly, for cases with presumptive cutaneous inoculation. 476 The neck and submandibular region are the most common sites.477.478.479. and 480. Scrofuloderma usually presents as an undermined ulcer or discharging sinus, with surrounding induration and dusky red discoloration.332.347.481. and 482. Sporotrichoid lesions were present in one case. 435 Internal organs are often involved in this form of the disease. 483 In contrast, patients with organ tuberculosis uncommonly have cutaneous disease.484. and 485. Non-tuberculous (atypical) mycobacteria are often responsible for this type of infection. It has been reported following BCG vaccination.379. and 380.
The epidermis is usually atrophic or ulcerated, an underlying abscess and/or caseation necrosis involving the dermis and subcutis. 347 At the periphery of the necrotic tissue there are granulomas. They have fewer lymphocytes than is usual in tuberculosis, suggesting a weak cell-mediated immune response. 486 Acid-fast bacilli can usually be found in smears taken from the affected area, although they are not always demonstrable in tissue sections. 332
Orificial tuberculosis is a rare form of cutaneous tuberculosis that presents as shallow ulcers at mucocutaneous junctions in patients with advanced internal (usually pulmonary) tuberculosis.357.487.488. and 489. It results from autoinoculation. 490 Perianal tuberculosis is a rare variant of orificial tuberculosis.491.492. and 493.
There is ulceration with underlying caseating granulomas and numerous acid-fast bacilli.
Disseminated cutaneous tuberculosis results from the dissemination of tubercle bacilli from pulmonary, meningeal, or other tuberculous foci, 494 particularly in children.478.495. and 496. There may be an underlying disturbance of the immune system predisposing to this widespread infection. 497The usual presentation is with papules, pustules, vesicles, and abscesses that become necrotic, forming small ulcers.338.347.497.498. and 499. Disseminated infection in patients with AIDS may take the form of multiple small pustules341 or erythematous papules. 500 As such, the cutaneous lesions may be a manifestation of more widespread miliary tuberculosis. 501
In early lesions there is focal necrosis and abscess formation, with numerous acid-fast bacilli, surrounded by a zone of non-specific chronic inflammation.347. and 497. In older lesions granulomas usually develop in this outer zone. In the pustular lesions seen in patients with AIDS, there are numerous neutrophils in the papillary dermis, with rare Langhans giant cells. 341
Tuberculids are a heterogeneous group of cutaneous lesions which occur in association with tuberculous infections elsewhere in the body, or in other parts of the skin,331.503.504. and 505. in patients with a high degree of immunity and allergic sensitivity to the organism. 506 Nevertheless, rare cases have been reported in patients with HIV infection. 507 Tuberculids may act as sentinel lesions of visceral tuberculosis, and their presence should initiate a search for overt or occult disease. 508 Tuberculids have also followed BCG vaccination. 378 Tuberculids are increasing in incidence in some countries. 509 Bacteria cannot be isolated from tuberculids, although M. tuberculosis DNA can be demonstrated in some cases using PCR.510. and 511. IGRAs (see above) have also detected latent tuberculous infection in tuberculids. 371 Response to antituberculous therapy has been recorded even in some cases in which no bacterial DNA could be detected.
The concept of tuberculids has been challenged from time to time, but they are usually considered to include erythema induratum–nodular vasculitis, lichen scrofulosorum, papulonecrotic tuberculid, and nodular tuberculid;350. and 352. granulomatous phlebitis is possibly a fifth type (phlebitic tuberculid),512. and 513. although there are some similarities to nodular tuberculid. More than one type of tuberculid is sometimes present,514. and 515. and transformation from one type into another has been recorded. 516 Sometimes the tuberculid does not fit neatly into one of the current categories. One such case resembled generalized granuloma annulare. 517
Erythema induratum–nodular vasculitis.
This is a panniculitis (see p. 463) which presents with bluish-red plaques and nodules, with a predilection for the lower part of the legs, particularly the calves.518. and 519. The coexistence of this condition and papulonecrotic tuberculid has been reported. 520M. tuberculosis DNA has been recovered from lesional skin using a PCR technique. 521
Lichen scrofulosorum.
This tuberculid is characterized by asymptomatic, slightly scaly papules measuring 0.5–3 mm in diameter.522.523.524.525.526. and 527. They are often follicular in distribution, mainly affecting the trunk of children and young adults.523. and 528. Localization to the vulva is a rare event.529. and 530. Lichen scrofulosorum usually occurs in association with tuberculosis of bone or of lymph nodes, but it may be associated with tuberculous infection in other sites;522.523.531. and 532. rarely it may follow BCG vaccination, 377 or infection with M. avium, 533 or other non-tuberculous mycobacteria. 534 It sometimes appears during treatment of tuberculosis. 535
Papulonecrotic tuberculid.
This presents as dusky papules or nodules that sometimes undergo central necrosis and which leave varioliform scars on healing.536.537.538. and 539. There is a predilection for the extremities, although the ears and genital region may also be involved.540.541.542. and 543. A variant with verrucous lesions mimicking Kyrle’s disease has been described. 544 It is uncommon in children. 545 In one study, a focus of tuberculosis was identified elsewhere in the body in 38% of cases.518.546. and 547. The tuberculin test is usually strongly positive and the lesions respond to antituberculous drugs. Multidrug resistant strains of tuberculosis have been encountered. 548 In a number of cases M. tuberculosis DNA can be demonstrated in lesional skin by PCR.545. and 549. Non-tuberculous (atypical) mycobacteria have also been implicated.550. and 551.
Erythema induratum–nodular vasculitis is a lobular panniculitis. It is not possible to diagnose which cases are due to tuberculosis on the histology alone (see p. 463). Traditionally, erythema induratum has been distinguished from nodular vasculitis on the basis of more prominent necrosis of the fat lobules and the extension of tuberculoid granulomas into the lower dermis.
Lichen scrofulosorum is characterized by non-caseating tuberculoid granulomas in the upper dermis; 555 the lesions have a perifollicular and eccrine localization. 523 Bacilli are not demonstrable.
Papulonecrotic tuberculid exhibits ulceration and V-shaped areas of necrosis, which include a variable thickness of dermis and the overlying epidermis. There is a surrounding palisade of histiocytes and chronic inflammatory cells, and an occasional well-formed granuloma.536. and 545. Vessels in the vicinity show disruption and fibrinoid necrosis of their walls, sometimes with accompanying thrombosis or vasculitis.340. and 556. Follicular necrosis or suppuration is present in approximately 20% of cases. 537 No bacilli can be demonstrated using routine staining methods. 536
In nodular tuberculid granulomatous inflammation is located at the junction of the subcutaneous fat and the lower dermis. It involves the walls of arterioles. 552
The treatment of cutaneous tuberculosis usually involves the concurrent use of four drugs – isoniazid, rifampicin, pyrazinamide, and either ethambutol or streptomycin – for a period of 8 weeks.352.421. and 483. This quadruple therapy is followed by a 16-week course of isoniazid and rifampicin. Variations in these schedules have been used successfully.456.464. and 473. Pyridoxine may be added to this protocol to prevent the neurotoxicity of isoniazid. 405 Drug-resistant strains are becoming more frequent. Drug-resistant tuberculosis is defined as disease resistant to the first-line drugs rifampicin and isoniazid, ± resistance to other drugs. 557 In India, organism resistance to isoniazid varies from 13 to 45%, and for streptomycin it is 3–12%. 557 In such cases, the treatment can be changed to ethambutol, ofloxacin, and thioacetazone. 557
Because there is sometimes difficulty in making and/or confirming the diagnosis of cutaneous tuberculosis, a therapeutic trial of antituberculous therapy is sometimes commenced. It is generally thought that 6 weeks of therapy with three or four antituberculous drugs is adequate to prove or disprove the diagnosis. 558
The non-tuberculous (atypical, environmental) mycobacteria are a heterogeneous group of acid-fast bacteria which differ from M. tuberculosis in their clinical and cultural characteristics, as well as in their sensitivities to the various antimycobacterial drugs.559. and 560. The traditional Runyon classification of the non-tuberculous (atypical) mycobacteria, which is based on colonial pigmentation, morphology, and growth characteristics, is of no relevance to dermatopathology.
Familial atypical mycobacteriosis (OMIM 209950) is a rare syndrome due to a constellation of genetic abnormalities, some of which have involved only a few families. The genes involved include the interferon-gamma receptor-1 and -2 genes (IFNGR1 and IFNGR2, respectively), the beta-1 chain of the interleukin-12 receptor (IL12RBI) gene, and the STAT-1 gene. 561
Non-tuberculous (atypical) mycobacteria can cause infections in the lungs, lymph nodes, meninges, synovium, and skin.559.562. and 563. Cervicofacial lymphadenitis due to non-tuberculous mycobacteria is not uncommon in children. 564 Two species, M. ulcerans and M. marinum, result in well-defined clinicopathological entities known respectively as Buruli ulcer and swimming pool (fish tank) granuloma. They merit individual consideration. Cutaneous infections are rarely produced by the other non-tuberculous (atypical) mycobacteria; when they occur they are associated with a diversity of clinical and histopathological appearances that are not species specific.330. and 565. Accordingly, they will be considered together after discussion of M. ulcerans and M. marinum infections.
Infections with Mycobacterium ulcerans (Buruli ulcer, Bairnsdale ulcer) have been reported from many areas of Central and West Africa,566. and 567. as well as from New Guinea, Australia, 568 southeast Asia, 569 and Mexico. The natural reservoir of the organism and the mode of transmission are unknown, 570 although current evidence suggests that infection is acquired through abraded skin after contact with contaminated water, soil, or vegetation.567. and 571. The organism has been identified by PCR in several cases of sea-urchin granulomas. 572 The organism grows preferentially at 32–33°C and produces an exotoxin which is responsible for tissue necrosis. 570 PCR testing can be used in tracing the source of the infection by means of a DNA fingerprinting method. 573
M. ulcerans produces lesions that predominantly involve the skin and subcutaneous fat, and less commonly the underlying fascia, muscle and, rarely, bone. Systemic spread is exceedingly rare. 574 The initial lesion is usually a papule or pustule on the extremities, particularly on the lower part of the legs. Ulceration soon develops and may extend quite rapidly. The ulcer is painless and has a characteristic undermined edge. 575 Satellite lesions may develop. 570 Affected individuals are usually children or young adults. 567 Patients with HIV infection may have aggressive infection. 576 Delay in presentation and time to diagnosis is often prolonged in non-endemic areas. 577
A consensus conference held in 2006 recommended surgical excision with a small rim of normal tissue as the treatment of choice. 578 Antibiotics play a role in countries in which surgery is not readily available, and in extensive disease. The WHO recommends the combination of oral rifampin (rifampicin) and parenteral streptomycin for initial treatment. Clarithromycin or ciprofloxacin may be added. 578 Treatment failure is less common if antibiotics are combined with surgery. 579
The most striking feature is extensive ‘coagulative’ necrosis involving the dermis and subcutaneous fat, with remarkably little cellular infiltration (Fig. 23.8). The necrosis is probably caused by a macrolide toxin called mycolactone. 581 A septolobular panniculitis is present in some areas. In the viable margins there is a mixed inflammatory cell infiltrate. The epidermis at the margins of the ulceration shows variable changes; pseudoepitheliomatous hyperplasia may sometimes be present. 582 There is sometimes a vasculitis involving small vessels in the septa of the subcutaneous fat adjacent to areas of fat necrosis. There are usually numerous clumps of acid-fast bacilli, sometimes forming globular structures, in the necrotic tissue. These organisms are extracellular in location.
Mycobacterium ulcerans. (A) There is necrosis of the dermis with peripheral extension beyond the limits of the surface ulceration (so-called ‘undermining’). An epidermal downgrowth is present at the edge of the ulcer. (B) There is suppuration and necrosis in the subcutis. (H & E)
In long-standing or recurrent lesions there is usually a granulomatous response, although the granulomas are poorly defined. Caseation is absent. Organisms tend to be sparse in these lesions.
Calcification sometimes occurs in the necrotic tissues in cases of long standing. 583
Mycobacterium marinum is a non-tuberculous (environmental, atypical) mycobacterium which has its natural habitat in brackish water, swimming pools, and aquarium tanks.584.585.586. and 587. In some areas it is the most common mycobacterial infection. 588 As it is unable to multiply at the temperature of the internal organs, the lesions that result from infection by it are usually confined to the skin. Involvement of the synovium and of the larynx589 has been reported.
The cutaneous lesions are usually solitary verrucoid nodules or plaques on the elbows, hands, or knees, which develop 1–2 weeks after superficial trauma, usually sustained in an aquatic environment.590. and 591. Laboratory inoculation has also been reported. 592 Lesions may persist for months or years if untreated. Such cases have been seen among South Pacific Islanders, in whom untreated lesions have developed into extensive warty and ulcerated plaques. 593 Sometimes, in the so-called ‘sporotrichoid’ form of infection, there are multiple lesions along the course of the main superficial lymphatic vessels, reminiscent of the ascending lymphangitis of sporotrichosis.584.594.595.596. and 597. Sporotrichoid lesions have been reported in a patient with Crohn’s disease receiving infliximab. 598 More extensive cutaneous involvement has been reported in immunosuppressed individuals,588. and 599. and in a child.600. and 601. Simultaneous infection with Nocardia asteroides has also been reported. 602
The second-generation tetracyclines, particularly minocycline, are frequently used to treat M. marinum infections. 603 Newer fluoroquinolones, including sparfloxacin, and moxifloxacin have also been used. In a recent report of 16 patients with M. marinum infections, the macrolide antibiotic clarithromycin was effective in all cases. 604
The inflammatory process is usually confined to the dermis, although in the sporotrichoid form subcutaneous involvement is more common. The changes are quite variable, and range from a mostly acute process with suppuration to granuloma formation. 607 Early lesion can be quite neutrophilic. The granulomas are usually poorly formed; there is no evidence of caseation. Some neutrophils may be admixed, in places forming suppurative granulomas. A chronic inflammatory cell infiltrate is present between the granulomas and foci of suppuration. Rarely, the pattern may simulate interstitial granuloma annulare. 608 Epidermal changes are variable and include ulceration, acanthosis, and sometimes even pseudoepitheliomatous hyperplasia (Fig. 23.9). Lichenoid changes were present in one case resulting in a lichenoid and granulomatous dermatitis. 609
Mycobacterium marinum. (A) There is marked acanthosis. (B) Granulomas are present in the lower dermis with intervening chronic inflammatory cells. (H & E)
Acid-fast bacilli are found in a minority of cases. 605 The organisms tend to be longer and fatter than M. tuberculosis, and sometimes they show transverse bands. 585 Rarely, organisms are abundant. 606 Isolation of the organism is readily achieved in culture at 30–33°C. PCR-based methods are now being used to confirm the diagnosis. 596 One of these involves examination of the hsp65 gene. 610
There is a miscellaneous group of non-tuberculous (atypical) mycobacteria. The M. fortuitum complex (M. fortuitum group and M. chelonae/abscessus group) account for the majority of skin and soft tissue infections in the United States. 611 They produce a diverse range of cutaneous lesions612 including solitary verrucous nodules, 613 erythematous nodules, 614 ulcers,615.616. and 617. furuncles, 611 abscesses, 618 cellulitis, 619 rosacea-like lesions, 620 disseminated suppurative panniculitis, 621 spindle-cell pseudotumors,581.622.623. and 624. acne conglobata-like lesions, 625 and sporotrichoid nodules.626.627.628.629. and 630. Infection may follow minor trauma, 631 injections, 632 including mesotherapy and acupuncture,633.634. and 635. body piercing, 636 tattooing,637. and 638. biopsy and surgical procedures,639.640. and 641. and the implantation of prostheses, or occur in immunosuppressed individuals.612.618.642. and 643. The species involved include M. kansasii,401.551.618.619.644.645.646.647.648.649. and 650. M. haemophilum,651.652.653.654.655.656.657.658. and 659. M. scrofulaceum,660. and 661. M. szulgai,628.662. and 663. M. xenopi, 604M. neoaurum, 664M. gordonae,604.613.622.665. and 666. the M. avium-intracellulare complex,498.667.668.669.670.671.672.673.674.675.676. and 677. M. fortuitum,614.640.678.679.680. and 681. M. abscessus,633.682.683.684.685. and 686. and M. chelonae (formerly M. chelonei).631.632.642.687.688.689.690.691.692.693.694. and 695. Unlike M. chelonae/abscessus infections that occur in compromised hosts, M. fortuitum disease most commonly occurs in healthy individuals.696. and 697. The advent of PCR has allowed M. avium to be distinguished from M. intracellulare but few reports have appeared using this new technique for this purpose. 533 PCR techniques can rapidly detect and speciate many mycobacteria.698. and 699. An increasing number of disseminated infections caused by the M. avium-intracellulare complex are being reported in patients with AIDS, although cutaneous lesions are uncommon in these cases.669.700.701.702. and 703. They are sometimes seen after the initiation of highly active antiretroviral therapy (HAART). 704 The appearance of skin lesions is thought to be a manifestation of partial immune restoration revealing a latent mycobacterial infection.705. and 706. Sporotrichoid lesions are a rare manifestation of infection with M. avium complex. 707 This organism has been reported, limited to the skin, in a patient with systemic lupus erythematosus. 708 It has also been isolated from lesional skin in a case of erythema multiforme. 709 Dissemination of a localized cutaneous infection can occur if immunosuppressive therapy is commenced mistakenly. 710
The treatment of M. ulcerans and M. marinum infections of the skin has already been discussed (see above). The treatment of other non-tuberculous mycobacteria should be informed by the antibiotic susceptibility results for the organism being treated. Clarithromycin is suitable for M. abscessus, M. gordonae, M. xenopi, M. scrofulaceum, and M. fortuitum.604. and 661. In the case of M. chelonae linezolid is sometimes added. 643 High levels of resistance to many antimycobacterial drugs are now being seen with M. chelonae infections.693. and 696. Amikacin is also used with clarithromycin for this organism as the use of two drugs reduces the incidence of drug-resistant strains. 633 Levofloxacin has been used for drug-resistant M. fortuitum infections, again with amikacin.681. and 696. Imipenem, quinolones, and sulfonamides are other drugs that can be used for this species.611. and 614. For M. haemophilum infections ciprofloxacin, clarithromycin, rifampicin, imipenem, and sulfamethoxazole have been used as monotherapy, or in dual combinations. 655
The changes are not species specific. They include tuberculoid granulomas, poorly formed granulomas, non-specific chronic inflammation, suppuration, and intermediate forms. 330 Less common patterns include a septal711 or mixed lobular and septal panniculitis, 330 a necrotizing folliculitis, 712 a lichenoid and granulomatous dermatitis, 609 and a diffuse histiocytic infiltrate in the dermis resembling lepromatous leprosy668. and 713. or the cells of Gaucher’s disease. 714 The presence of poorly formed granulomas with intervening chronic inflammation and foci of suppuration should always raise the suspicion of an infection by atypical mycobacteria. Suppurative inflammation with microabscess formation and pseudocyst formation with numerous acid-fast bacilli is a characteristic feature of infections caused by rapid-growing species. 715 The location of the inflammation is inconstant, with no specific relationship to the organism or the type of inflammation. 330 The inflammation is mostly centered on the dermis, with some extension into the subcutis; the latter is more likely in immunosuppressed patients. 712
Infections caused by M. haemophilum are posing an increasing diagnostic problem. They may present with non-granulomatous or pauci-granulomatous reactions without necrosis. A mixed suppurative and granulomatous reaction is still the most common pattern seen with this organism, but lichenoid (interface) and non-specific perivascular inflammation as well as necrotizing lymphocytic vasculitis are sometimes reported. 653
In cases with spindle-cell pseudotumors there are plump to spindle-shaped cells with a histiocytic to myofibroblastic appearance.622.624.716. and 717. The stroma contains collagen bundles of variable thickness. Acid-fast bacilli may be quite numerous within the spindle cells and epithelioid histiocytes. 717 The spindle cells stain for CD68 and sometimes desmin, but not for factor XIIIa. 718
Organisms are difficult to find, even in preparations stained to demonstrate acid-fast bacilli. They may be plentiful in cases with a histiocytoid pattern and in some infections with M. avium, M. abscessus, M. chelonae, and M. intracellulare, although they are rarely as plentiful as in infections with M. ulcerans. The bacilli of M. kansasii are larger, broader and more coarsely beaded than other mycobacteria. 581 Grains formed of bacillary clumps and somewhat resembling those seen in mycetomas have been reported in a case of M. chelonae infection. 687 Intracellular non-tuberculous (atypical) mycobacteria have been said to be PAS positive, according to one report. 719 It should be noted that Vibrio extorquens, a rare cause of skin ulcers, is weakly acid fast in tissue sections; 720 granulomas may also be present. Also in this category (skin lesions with granulomas and acid-fast bacilli) are the lesions caused by Rhodococcus. 721
Leprosy (lepra) is a chronic infection caused by Mycobacterium leprae. It affects mainly the skin, nasal mucosa, and peripheral nerves. Leprosy is most prevalent in tropical countries, particularly India, southeast Asia, Central Africa, and Central and South America, particularly Brazil. In India, which has two-thirds of the global leprosy burden, the case detection rate has remained stubbornly around 5 per 10 000 population. 722 Of the 122 countries in which leprosy was considered endemic, 107 had achieved the ‘elimination target’ (1 case per 10 000 population) by the year 2002. 723 Leprosy is uncommon in Europe and the United States, where it occurs in persons from other countries; diagnosis is sometimes delayed.724. and 725. In 2001, there were 6518 active cases of leprosy registered in the United States. 723 There appears to be an endemic focus of leprosy in Texas, around the Gulf Coast. 726 Leprosy is also rare in Australia, with an average of eight cases per year over the last 5 years. 727 Approximately half of these cases occur in the indigenous population, with most other cases in persons from endemic countries. 727
M. leprae is an obligate, intracellular, Gram-positive organism which is also acid fast, though less so than M. tuberculosis. The organism cannot be grown in vitro, although claims of successful cultivation are made from time to time. 728M. leprae can be grown in the footpads of mice and in the nine-banded armadillo, an animal in which natural infection also occurs. The sooty mangabey monkey is another host. 729M. leprae shares many antigens with other mycobacteria; it also possesses a unique antigen, phenolic glycolipid-1 (PGL-1).730. and 731. Experimental studies are currently attempting to determine the exact role of the various components of the immune system in the defense against this organism. 732M. leprae is found predominantly in three main cell types in the skin – Schwann cells, endothelial/perithelial cells, and cells of the monocyte–macrophage system. 733
The incubation period of leprosy is not known with certainty, but is thought to average 5 years. 730 This uncertainty and the long period make any study of the mode of transmission difficult. The organism is of low infectivity, and prolonged and/or close contact with patients who have the disease is considered necessary for transmission to occur. 734 The incidence of conjugal leprosy is low, further testimony to its low infectivity. 735 Contact with the armadillo may be an important source of infection in some countries.736. and 737. There may also be a genetic predisposition to the disease. 738 The HLA genotypes HLA-DR2 and -DQ1 appear to increase personal susceptibility to leprosy. 739 A strong leprosy susceptibility factor has been linked to chromosome 6q25–q26 and a further locus is at 6p21. 740 A low-producing lymphotoxin-α allele has been identified as a major risk factor for early-onset leprosy. 740 The portal of entry of the organism is not known, but the skin and upper respiratory tract, particularly the nasal mucosa, are probable sites. 741 Droplet infection is the most likely mode of transmission, although rare cases of inoculation infection have been recorded,741. and 742. particularly from tattooing. 743
Leprosy exhibits a spectrum of clinical characteristics that correlate with the histopathological changes and the immunological status of the individual.730.733.746.747.748. and 749. At one end of the spectrum is tuberculoid leprosy (TT), which is a highly resistant form with few lesions and a paucity of organisms (paucibacillary leprosy). 750 At the other end is lepromatous leprosy (LL), in which there are numerous lesions with myriad bacilli (multibacillary leprosy), and an associated defective cellular immune response.750. and 751. This response may, in part, result from a genetic alteration in the IL-12 receptor β2 gene, leading to a reduced production of interferon-γ by Th1 lymphocytes. 752 In between these poles there are clinical forms which are classified as borderline-tuberculoid (BT), borderline (BB), and borderline-lepromatous (BL) leprosy. The clinical state of an individual may alter along this scale, although the polar forms (TT and LL) are the most stable and the borderline form (BB) the most labile. This categorization, known as the Ridley–Jopling classification,747. and 753. is often modified by the addition of subpolar forms at either end of the spectrum (TTs and LLs), giving additional categories of subpolar lepromatous leprosy and subpolar tuberculoid leprosy. In the author’s view this is taking ‘splitting’ to a level that is difficult to justify. There is also an indeterminate form of leprosy, which is classically the first lesion to develop although it may be overlooked clinically.
Indeterminate leprosy is most often recognized in endemic regions. 754 It presents as single or multiple slightly hypopigmented or faintly erythematous macules, usually on the limbs. 755 Sensation is normal or only slightly impaired. Indeterminate lesions often heal spontaneously, but in approximately 25% of cases this form develops into one of the determinate types, usually in the borderline region of the spectrum. 735 The clinical features of the determinate forms will now be considered in further detail.
Tuberculoid leprosy (TT) is a relatively stable form and the most common type in India and Africa. 735 There are one or several sharply defined reddish-brown anesthetic plaques which are distributed asymmetrically on the trunk or limbs. 756 An enlarged nerve may be seen entering and leaving the plaque; other superficial nerves, such as the ulnar and popliteal nerves, may also be enlarged, leading to nerve palsy. 756 Giant nerve ‘abscesses’ may occur.757. and 758. The lepromin test is positive. This category is often divided into polar and subpolar forms. The T helper (Th) type 1 cytokines, principally interleukin (IL)-2 and interferon-γ, are more strongly expressed in tuberculoid leprosy than in other types. 759
Borderline-tuberculoid leprosy (BT) is usually associated with more numerous lesions than tuberculoid leprosy, and these are usually smaller. Daughter (satellite) patches may develop. Hypoesthesia and impairment of hair growth within the lesions are often present. 735
Borderline leprosy (BB) is an unstable form with erythematous or copper-colored infiltrated patches which are often annular. Margins are often ill defined.
Borderline-lepromatous leprosy (BL) is associated with more numerous lesions that are less well defined, more shiny, and less anesthetic than those at the tuberculoid end of the spectrum. Nodular lesions resembling those seen in lepromatous leprosy may be present. A rare subepidermal bullous form has been reported. 760 Rarely, it may present as a solitary cutaneous plaque. 761 Nerve involvement, without cutaneous lesions other than hypoesthesia, has been seen.762. and 763.
Lepromatous leprosy (LL) usually develops from borderline or borderline-lepromatous forms by a downgrading reaction. Polar and subpolar forms have been recognized. It is a systemic disease, although the primary clinical manifestations are in the skin. 764 Mucosal involvement may lead to ulceration of the nasal septum, whereas nerve lesions may result in acral anesthesia, claw hand, and foot drop. 735 The cutaneous lesions, which are usually symmetrical, include multiple small macules, infiltrated plaques, and nodules with poorly defined borders. Multiple facial nodules give a bovine appearance, and this is usually accompanied by sparse eyebrows. 735 Various autoantibodies have been demonstrated in this form of leprosy. 765 This may explain the increased incidence of vitiligo. 766 Cryoglobulinemia has been reported in up to 95% of cases. It may result in leg ulcers. 767 Th2 cytokines, notably IL-4, IL-5, and IL-10, are characteristic of this form of leprosy. 759 There is a high expression of COX-2 in LL. 768 Experimental attempts are being made to culture, ex vivo, macrophages from patients with lepromatous leprosy to understand better the mechanism of this specific anergy in these patients. 769
In cases of lepromatous leprosy the diagnosis can be confirmed by PCR amplification of the mycobacterial heat shock 65 gene (Hsp65) from DNA extracted from skin lesions.770. and 771.
Histoid leprosy is a rare variant of the nodular variety of lepromatous leprosy, and is characterized by the presence of cutaneous and/or subcutaneous nodules and plaques on apparently normal skin.772.773.774.775.776. and 777. It may be caused by a drug-resistant strain of M. leprae, as it usually develops in cases of long standing. 773 It may also develop in untreated lepromatous leprosy. 778
Although only a few instances of leprosy have been reported in patients with AIDS, the long incubation period and its occurrence in areas of Central Africa where AIDS is also endemic suggest that leprosy complicating AIDS may be a problem in the future.779.780.781. and 782.
Reactional states are acute episodes that interrupt the usual chronic course and clinical stability of leprosy.785. and 786. They are expressions of immunological instability. Pregnancy, which causes a relative decrease in cellular immunity, may precipitate a reactional state. 787 They may also occur as a manifestation of the immune reconstitution inflammatory syndrome seen in patients with HIV treated with highly active antiretroviral therapy (HAART). 788 The reactions, which are major causes of tissue injury and morbidity, have been subdivided into three variants: the lepra reaction (type I), erythema nodosum leprosum (type II), and Lucio’s phenomenon (sometimes included with type II reactions, at other times designated type III).785. and 789. Not included in these categories, but nevertheless a manifestation of an upgrading reaction, is the rare sporotrichoid pattern caused by spread along peripheral nerves. 790 RAGE (receptor for advanced glycation end-products), a member of the immunoglobulin superfamily, and its ligand EN-RAGE are involved in triggering the release of key inflammatory mediators. They appear to be involved in the proinflammatory process, especially during the lepra reaction. 791 The expression of B7, on the surface of antigen-presenting cells, precedes reactional episodes in patients with multibacillary leprosy. 792
Lepra (type I) reactions occur in borderline leprosy, and are usually associated with a shift toward the tuberculoid pole (upgrading) as a result of an increase in delayed hypersensitivity. It is an example of a type IV cell-mediated reaction. Uncommonly the shift is toward the lepromatous pole (a downgrading reaction).786. and 793. Lepra reactions are often seen in the first 6 months or so of therapy, but they may occur in untreated patients or be associated with pregnancy, stress, or intercurrent infections. 794 It may follow the completion of therapy. 795 They are characterized by edema and increased erythema of skin lesions, sometimes accompanied by ulceration and the development of new lesions. Constitutional symptoms and neuritis may be present. 796
Erythema nodosum leprosum (type II reaction) occurs in 25–70% of all cases of lepromatous leprosy,785. and 797. and occasionally in association with the borderline-lepromatous form.798.799. and 800. There are crops of painful, erythematous, and violaceous nodules, particularly involving the extremities. 801 The occurrence of bullous lesions is exceedingly rare. 802 Individual lesions last 7–20 days. 803 Severe constitutional symptoms may be present.783. and 803. The pattern varies somewhat in severity in different ethnic groups, 789 necrotizing lesions sometimes developing. 804 Type II reactions are the result of an immune complex-mediated vasculitis, 798 although the participation of certain cytokines indicates that some cell-mediated components are involved. 805
Lucio’s phenomenon (erythema necroticans) is seen mainly in Mexicans with the diffuse non-nodular form of lepromatous leprosy.803.806. and 807. It is rare in other ethnic groups.808.809.810. and 811. Hemorrhagic ulcers form as a result of the underlying necrotizing vasculitis812 or of vascular occlusion due to endothelial swelling and thrombosis. 806 Some cases are fatal. 809
Multiple drug therapy has now been used for many years in the treatment of leprosy to address dapsone resistance, to reduce the length of therapy (resulting in improved compliance), and to keep rifampin (rifampicin) in all therapeutic regimens because of its effectiveness, even when taken once a month. 723 The current WHO recommended treatment is as follows: 723
1. For paucibacillary disease, 600 mg rifampin monthly and 100 mg dapsone daily with six cycles in 9 months;
2. For multibacillary disease, 600 mg rifampin and 300 mg clofazimine monthly and 100 mg dapsone and 50 mg clofazimine daily with 24 cycles in 36 months or 12 cycles in 18 months;
3. For single paucibacillary lesions (usually indeterminate leprosy) one dose of ROM (rifampin 600 mg, ofloxacin 400 mg, minocycline 100 mg). Treatment with six once-monthly doses of ROM has been used to treat borderline tuberculoid leprosy in a woman with armadillo exposure. 737
Anti-leprosy vaccination can be immunoprophylactic or immunotherapeutic. It is used in high-incidence countries such as India. 723
In type I reactional states, systemic corticosteroids are necessary if neuritis is present. In milder cases, the non-steroidal anti-inflammatory drugs (NSAIDs) can be used.723. and 794. In type II reactions, corticosteroids are used if neuritis is present. In the absence of neuritis, drugs such as thalidomide, colchicine, and pentoxifylline can be used. 723 Steroids and the addition of an augmented clofazimine dose should be added to the WHO schedule in cases of Lucio’s phenomenon. 807
Skin biopsies should include the full thickness of the dermis and should be taken from the most active edge of a lesion. The bacilli may be detected in tissue sections using the Fite–Faraco staining method or an immunofluorescent technique. 813
Two distinct types of histological changes are found in leprosy: the lepromatous reaction, in which large numbers of macrophages in the dermis are parasitized with acid-fast bacilli (Fig. 23.10), and the tuberculoid reaction, in which there are tubercle-like aggregates of epithelioid cells, multinucleate giant cells, and lymphocytes, bacilli being difficult to find. These histological patterns are seen at either end of the clinical spectrum of leprosy; features overlapping with those of borderline forms may be present. Sometimes there is a disparity between the clinical and the histological subclassification of leprosy. 814 Early lesions are mostly of indeterminate or tuberculoid type. 815
Lepromatous leprosy. Numerous acid-fast bacilli are present within macrophages and lying free in the dermis. (Wade–Fite)
Indeterminate leprosy is characterized by a superficial and deep dermal infiltrate around blood vessels, dermal appendages and nerves, composed predominantly of lymphocytes with a few macrophages (Fig. 23.11). 747 Mast cells are markedly increased in number and they may extend into the small nerves in the deep dermis. 816 Less than 5% of the dermis is involved by the infiltrate. 816 Mild proliferation of Schwann cells may occur, but marked neural thickening is quite uncommon. 755 The term neuritic leprosy is used if there is an inflammatory infiltrate in any layer of the nerve fascicle, and one or more acid-fast bacilli in the nerve. 817 There may be a few lymphocytes in the epidermis; this is one of the earliest changes to occur in leprosy. 815 Bacilli are usually quite difficult to find, 815 but if serial sections are cut and a small dermal nerve is followed, sooner or later a single bacillus or a small group of bacilli will be discovered. 754 The mechanism of the hypopigmentation in this and other forms of leprosy is still not clear. There is a reduction in melanin in the basal layer; in some reports a decreased number of melanocytes has also been recorded. 818
(A) Indeterminate leprosy. (B) There is a superficial and deep infiltrate of lymphocytes with periappendageal and perineural extension. Epithelioid cells are present. (H & E)
Tuberculoid leprosy (polar and subpolar) shows a tuberculoid reaction throughout the dermis, with non-caseating granulomas composed of epithelioid cells, some Langhans giant cells, and lymphocytes (Fig. 23.12). 756 The predominant lymphocyte present is the T-helper cell, which is found throughout the granuloma, whereas T-suppressor cells predominate in the lymphocyte mantle that surrounds the granulomas.819. and 820. There are said to be fewer lymphocytes in the subpolar form, a rather tenuous feature on which to base a distinct category. Granulomas may erode the undersurface of the epidermis and they may also extend into peripheral nerves and into the arrectores pilorum. 756 Lepromatous phlebitis is rarely seen in this form of the disease. 821 Vascular involvement is more common in disseminated disease. The S100 stain is superior to H & E in identifying nerve fragmentation in tuberculoid leprosy. 822 Inflammation often engulfs the sweat glands. 815 Bacilli are found in less than 50% of cases. 756 Perineural granulomas may persist for at least 18 months after the cessation of effective therapy. 823
Tuberculoid leprosy. (A) Non-caseating granulomas extend throughout the dermis. (B) A deep cutaneous nerve contains a granuloma. (H & E)
Borderline-tuberculoid leprosy differs from tuberculoid leprosy in several ways. 735 Tubercle formation may be less evident and nerve destruction is not as complete. A subepidermal grenz zone is invariably present. Lymphocytes and Langhans cells are usually not as plentiful in the granulomas as in tuberculoid leprosy.
In borderline leprosy there are collections of epithelioid cells without the formation of well-defined granulomas. Langhans giant cells are absent and lymphocytes are more dispersed. Occasional bacilli can be found.
In borderline-lepromatous leprosy there are small collections of macrophages rather than epithelioid cells. These large cells have abundant granular cytoplasm, although a few may have foamy cytoplasm as seen in lepromatous leprosy. 735 A variable number of lymphocytes are present, and some of these are in the vicinity of small dermal nerves. A grenz zone is present in both borderline-lepromatous and true lepromatous leprosy. Bacilli are easily found.
Lepromatous leprosy (polar and subpolar) is characterized by collections and sheets of heavily parasitized macrophages within the dermis, with a sparse sprinkling of lymphocytes, the majority of which are CD8+ (Fig. 23.13). 820 Rarely, subcutaneous and deep dermal inflammatory nodules are present. 824 In older lesions the macrophages have a foamy appearance (lepra cells, Virchow cells). Numerous acid-fast bacilli are present in macrophages, sweat glands, nerves, Schwann cells, and vascular endothelium, 825 a finding which has been confirmed by electron microscopy. 826 An increase in angiogenesis also occurs. 827 Marked melanin incontinence is a rare finding. 828 The macrophages express S100 protein. 829 The organisms in the macrophages may be arranged in parallel array, forming clusters, or in large masses known as globi (Fig. 23.14). In the subpolar form, there are fewer globi and a few more T lymphocytes than in the polar form. 829 If normal-appearing skin is biopsied in leprosy, more than half of the cases will show histological changes of leprosy. This is more likely at the lepromatous end of the spectrum.830.831. and 832. A neuritic phase often precedes the development of visible cutaneous lesions. 832
Lepromatous leprosy. This case had numerous parasitized foamy macrophages with masses of organisms. (H & E)
Lepromatous leprosy with masses of organisms (globi) in the cytoplasm of macrophages. (H & E)
In histoid leprosy there are circumscribed nodular lesions consisting predominantly of spindle-shaped cells with some polygonal forms. The cells are arranged in an intertwining pattern.772. and 773. Some cases may resemble a fibrohistiocytic tumor (Fig. 23.15). Small collections of foamy macrophages may also be present. The component cells contain numerous acid-fast bacilli, which are longer than the usual bacilli of leprosy and are aligned along the long axis of the cell. 773 The spindle-shaped cells are dermal dendritic cells expressing factor XIIIa. 829 CD68-positive macrophages are also present. 833
(A) Histoid leprosy. (B) The cells mimic a fibrohistiocytic tumor. (H & E)
Lepra (type I) reactions show edema, an increase in lymphocytes and sometimes giant cells, as well as the formation of small clusters of epithelioid cells.834. and 835. Edema also occurs in the epithelioid cell granulomas. 836 In severe cases fibrinoid necrosis may be present, and this is followed by scarring. 793 This is the picture seen in the usual upgrading reaction. In downgrading reactions there is replacement of lymphocytes and epithelioid cells by collections of macrophages, and there is a corresponding increase in the number of bacilli. 793
Erythema nodosum leprosum is characterized by edema of the papillary dermis and a mixed dermal infiltrate of neutrophils and lymphocytes superimposed on collections of macrophages. 798 Bullous lesions are sometimes seen.802. and 837. There are relatively more T lymphocytes of helper-inducer type (CD4+) than in non-reactive lepromatous leprosy.819. and 838. This appears to be due to an absolute reduction in the number of T-suppressor (CD8+) cells, 839 which predominate in lepromatous leprosy. 840 A vasculitis is often present.798.803. and 840. There may be involvement of the subcutis, with the development of a mixed lobular and septal panniculitis; however, in the majority of cases involvement of the dermis is the primary and predominant finding. 804 Macrophages in the dermis contain fragmented organisms. 841 Direct immunofluorescence reveals the presence of C3 and IgG in the walls of dermal blood vessels. 798
Lucio’s phenomenon is usually the result of a necrotizing vasculitis of the small vessels in the upper and mid dermis, with associated infarction of the epidermis. 803 At other times there is prominent endothelial swelling and thrombosis of superficial vessels without a vasculitis. 806 The endothelial cells and macrophages in the dermis contain numerous bacilli.803. and 808. There is usually only a mild inflammatory infiltrate, with fewer neutrophils than in erythema nodosum leprosum. 808
Some of the organisms to be considered in this miscellaneous category have received attention recently as possible biological agents of terrorism. Because it is easily disseminated anthrax tops the list of potential organisms. Others include tularemia, the plague (Yersinia pestis), brucellosis, and psittacosis. 842
Anthrax is a zoonosis that primarily affects grazing herbivorous animals. 843 Humans are incidental hosts. It is mainly an occupational disease occurring in those who handle hair, wool, hide, or the carcasses of infected animals.844.845.846. and 847. The ritual of smearing cow’s blood on the forehead is another mode of transmission of anthrax to children. 848 The causative organism, Bacillus anthracis, may enter the body by the skin or by inhalation or ingestion. 849 Anthrax is now an exceedingly rare infection. 850 It has the potential to be used as an agent of bioterrorism. 842 Anthrax poses a potential threat for laboratory workers. 851 It can exist for decades as environmental spores.
The initial cutaneous lesion is usually on an exposed site. It begins as a papule, which soon becomes bullous and then ulcerates, with the formation of a hemorrhagic crust (eschar). 852 A ring of satellite vesicles may form. Extensive lesions have been reported in a diabetic patient. 853 The pathogenesis of anthrax is related to the production of exotoxins: edema toxin and lethal toxin. 842 They may result in a necrotizing vasculitis. Hemorrhagic lymphadenopathy, constitutional symptoms, and even a fatal septicemia may accompany the cutaneous lesion.
Vaccines are available, and treatment/prophylaxis with antibiotics is effective when started early. 842 Penicillin used to be the drug of choice, 854 but β-lactamase-producing strains of organism have been encountered. Ciprofloxacin and amoxicillin have been used to treat anthrax. 855 Doxycycline and one or two additional antimicrobials have also been recommended. 856
There is necrosis of the epidermis and upper dermis, with surrounding edema, fibrin extravasation, and hemorrhage. The blood vessels are conspicuously dilated. Fibrin thrombi are sometimes present. Inflammatory cells are often surprisingly sparse, although sometimes a florid cellulitis is present with focal abscess formation. 845 Spongiosis and vesiculation may occur in the epidermis adjacent to the ulceration.
B. anthracis can be seen, usually in large numbers, in the exudate. It is easily recognized by its large size, square-cut ends, and a tendency to be in chains. It is Gram positive. If antibiotic therapy has been given, organisms are sparse and often do not stain positively by the Gram procedure.
Brucellosis is a re-emerging disease in some countries. It is transmitted primarily from farm animals to humans. 561 Cutaneous involvement occurs in 5–10% of patients with brucellosis. 858 It takes the form of a disseminated papulonodular eruption or an urticarial maculopapular rash.858.859. and 860. Occasionally erythema nodosum-like nodules develop.561. and 860. Skin lesions may not develop for many weeks after the onset of symptoms of brucellosis. 858 A dermatitis has also been reported on the forearms of veterinarians involved in the manual removal of placentas from aborting cows infected with Brucella abortus. 859
The maculopapular lesions usually have a non-specific appearance with a mild perivascular infiltrate of lymphocytes in the upper dermis. 859 The papulonodules have an infiltrate of lymphocytes and histiocytes, which is both perivascular and periadnexal. There is also focal granulomatous change; multinucleate giant cells are uncommon. Focal exocytosis of inflammatory cells and keratinocyte necrosis may also be present. A leukocytoclastic vasculitis is exceedingly rare. 861
The erythema nodosum-like lesions show a septal panniculitis, with considerable extension of the infiltrate into the fat lobules accompanied by focal necrosis. Plasma cells are also abundant. These features are not usually associated with lesions of typical erythema nodosum. A lobular panniculitis with vasculitis has also been reported. 561
The treatment of brucellosis is variable. Oral doxycycline and intramuscular streptomycin were successful in one report. 561
Yersiniosis is a generally accepted term for diseases caused by Yersinia pseudotuberculosis and Y. enterocolitica. The incidence of cutaneous manifestations in yersiniosis is not known, although in some countries the disease is responsible for 10% or more of cases of erythema nodosum.862. and 863. Erythema multiforme and figurate and non-specific erythemas may also occur.862. and 864.
As in brucellosis, the erythema nodosum-like lesions may show some extension of the infiltrate into the fat lobules, as well as a necrotizing vasculitis and some necrosis. 862 The appearances in some cases are more in keeping with erythema induratum–nodular vasculitis than with erythema nodosum.
Granuloma inguinale (granuloma venereum, donovanosis) is a sexually transmitted disease caused by a small Gram-negative bacillus, Calymmatobacterium granulomatis. 865 It has a relatively low level of infectivity. Clinically, the external genitalia are involved in most cases866. and 867. but occasionally anal, vaginal, oral, or extragenital cutaneous involvement is found.868. and 869. The disease begins after an incubation period of from 2 to 4 weeks. The initial manifestation is a papule, which soon ulcerates. The lesion slowly spreads and may cause extensive destruction of the penis or vulva.870.871.872. and 873. It may also spread to involve the adjacent parts of the thighs or lower abdomen. 868 An association with HIV infection has been recorded. 873 Squamous cell carcinoma may supervene, but care should be taken not to misinterpret the marginal pseudoepitheliomatous hyperplasia as carcinomatous. 868
The diagnosis is easily made by examination of tissue smears stained by the Wright or Giemsa methods. Smears will show large mononuclear cells containing a number of the organisms, which appear as red-stained intracytoplasmic encapsulated ovoid structures with prominent polar granules. The organism can be cultured in an egg-yolk medium, and also by inoculation of the yolk sac of chick embryos. PCR-based tests are now available for specific diagnosis. 869
The quinolone antibiotics have been used to treat the disease, but other antibiotic categories can be used. 869
The center of the lesion is usually ulcerated, and at the margins there is often well-marked pseudoepitheliomatous hyperplasia. Plasma cells and macrophages predominate in the base of the ulcer, and there are often scattered neutrophil microabscesses as well. Blood vessels are prominent in the ulcer base, and sometimes endothelial swelling is present. 874 Transepithelial elimination of macrophages with cytoplasmic organisms has been reported through areas of pseudoepitheliomatous hyperplasia. 875 The diagnosis is made by finding parasitized macrophages. These are large cells, measuring 20 µm in diameter. Organisms are seldom numerous, but they can be recognized – if with some difficulty – in hematoxylin and eosin sections. They are more readily found in silver preparations, such as the Warthin–Starry method (Fig. 23.16), or when stained with Giemsa’s solution. Plastic-embedded semithin sections enhance diagnosis. 876 In these sections, and in smears, the vacuolated nature of the cytoplasm of the parasitized macrophages can be appreciated. The organisms (Donovan bodies) are present singly or in clusters in the vacuoles (Fig. 23.17). They measure 1–2 µm in diameter, and show bipolar staining in silver preparations. For this reason they have been likened to minute safety pins. There may be up to several dozen in a macrophage.
Granuloma inguinale. (A) The infiltrate consists of neutrophils, plasma cells, and parasitized macrophages. (H & E) (B) The organisms can be seen in the cytoplasm of macrophages in this silver preparation. (Warthin–Starry)
Imprint from a vulval lesion of granuloma inguinale. Numerous organisms are present in vacuoles in the cytoplasm of a macrophage. (Giemsa)
The organism, which has a prominent capsule but no flagella, may be seen within phagosomes of macrophages. 877
Chancroid is a sexually transmitted disease (STD) caused by the Gram-negative bacillus Haemophilus ducreyi.878.879.880.881. and 882. It has a short incubation period and presents as painful, irregular, non-indurated single or multiple ulcers, usually in the genital area or perineum.883.884. and 885. Sometimes the ulcers are on the lower legs. 886 Clinically, the ulcers may sometimes be difficult to distinguish from granuloma inguinale, or even herpes simplex.885. and 887. Approximately half of the patients have tender inguinal lymphadenopathy, and occasionally draining sinuses arise from the suppurating nodes.
Chancroid is now seen less frequently than previously in Western countries, usually occurring as limited, but significant outbreaks.888.889.890. and 891. The main reservoir for disease transmission is prostitutes. 892 However, it is a major cause of genital ulceration in Africa, where it appears to facilitate the heterosexual spread of HIV.893. and 894. It has been estimated that the probability of transmitting chancroid from an infected to an uninfected person during a single sexual exposure is 0.35. 895 Gram-negative rods forming parallel chains (‘school of fish’ appearance) may be seen in smears. Selective agars are also available that facilitate the rapid isolation of H. ducreyi.896. and 897.
Treatment with erythromycin or ciprofloxacin can be used to treat chancroid. In some countries broad-spectrum antibiotics are used to treat concurrent STDs. Intramuscular benzathine penicillin is sometimes curative. 886 Azithromycin and ceftriaxone can also be used. 895
There is usually a broad area of ulceration, in the base of which three zones can be discerned. Superficially there is tissue necrosis, fibrin, red cells, and neutrophils. Beneath this is a zone of vascular proliferation, with prominent endothelial cells and a mixed inflammatory infiltrate. In the deepest zone the infiltrate is more chronic, with plasma cells and lymphocytes predominating. The epidermis adjacent to the ulcer shows acanthosis, some overlying parakeratosis and occasional intraepidermal microabscesses.
A Giemsa stain or a silver impregnation method shows organisms both singly and in short chains, lying free and within histiocytes in the base of the ulcer.
Rhinoscleroma is a chronic inflammatory disease of the nasal and oral mucosa caused by the Gram-negative bacillus Klebsiella rhinoscleromatis. 898 In advanced cases it may spread to involve the larynx, trachea, bronchi, and lips. 899 Rhinoscleroma is endemic in parts of Africa, Asia, and Latin America. It is spread by direct or indirect contact. The incubation period is long. 898 Rhinoscleroma is an opportunistic infection that can occur in patients infected with HIV. 900
Initially there is a non-specific rhinitis with the development of a purulent nasal discharge and some hypertrophy of the inflamed mucous membrane. 898 This is followed by an infiltrative and nodular stage, during which the involved tissues become swollen and eventually deformed. 899 Obstruction of the nasal cavity can be caused by the friable inflammatory masses. There is scarring and further deformity in the late stages. That is, there are three stages of development: rhinitic, infiltrative, and cicatricial stages. 901
It is rare for spontaneous cure to occur, and accordingly, prolonged treatment with antibiotics is required. 901 The tetracyclines, fluoroquinolones, gentamicin, and trimethoprim-sulfamethoxazole have all been used at different times.901. and 902.
In the fully developed lesions of rhinoscleroma the changes are diagnostic, with a variable admixture of plasma cells with conspicuous Russell bodies, and large mononuclear cells with vacuolated cytoplasm (Mikulicz cells) (Fig. 23.18). In addition there are some lymphocytes and foci of neutrophils. The Mikulicz cell, which varies from 10 to 100 µm in diameter, is a macrophage. 903 The causative organisms may be seen in the cytoplasm of these cells in PAS and Gram preparations. However, they are best visualized with silver impregnation methods, particularly the Warthin–Starry stain (Fig. 23.19). 904 The organisms can also be seen in smears from the lesions, and they are easily cultured.
(A) Rhinoscleroma. (B) The inflammatory infiltrate consists of an admixture of plasma cells and vacuolated macrophages (Mikulicz cells). (H & E)
Rhinoscleroma. The organisms can be seen on this silver stain. (Warthin–Starry)
The dense inflammatory infiltrate may cause ulceration or atrophy of the overlying mucosa. Sometimes the mucosa is hyperplastic, even to the extent of pseudoepitheliomatous hyperplasia. 904
The vacuolated Mikulicz cells are macrophages with phagosomes containing bacterial mucopolysaccharide as well as some bacteria. 903 There is often fragmentation of the limiting membrane of some of the phagosomes. 903 Plasma cells are sometimes vacuolated to a limited extent. Russell bodies are readily seen in the plasma cells and extracellularly.
Tularemia is named after Tulare County, California, where its occurrence was first recognized. 905 It is caused by Francisella tularensis, a minute Gram-negative coccobacillus which produces a fatal insect-borne septicemic infection in ground squirrels and other rodents. The disease is transmitted to humans either by the bite of an infected vector, such as mosquitoes or ticks, 906 or by contact with an infected rodent or rabbit.905. and 906. It has been recognized in parts of the United States,907. and 908. Canada, 909 and Russia, and rarely in the British Isles and other parts of Europe. Skin lesions occur only in the ulceroglandular form of the disease. 910 This is characterized by a papule at the site of the initial inoculation, followed by an ascending lymphangitis. Nodules develop along the course of the affected lymphatics, as in the lymphangitic type of sporotrichosis. 905 The occurrence of erythema nodosum and erythema multiforme has been described. 911 Severe constitutional symptoms are usual. 912
Streptomycin is the drug of choice, but other aminoglycosides can also be used, as can tetracyclines, chloramphenicol, third-generation cephalosporins, rifampin (rifampicin), and erythromycin. 913 A human live vaccine has been developed; it is currently undergoing investigations. 914
The primary lesion is an ulcer, with necrosis involving the epidermis and upper dermis. The adjacent epidermis is usually acanthotic with some spongiosis. In acute lesions there is focal necrosis and suppuration, whereas in more chronic lesions a granulomatous picture has been described around the necrotic focus in the dermis.
The causative organism cannot be seen in conventional histological preparations. It can be demonstrated in histological sections and in films of exudate by direct immunofluorescence, following treatment with fluorescein-labeled specific antiserum. 907F. tularensis is a hazard to laboratory workers. 908
Listeriosis is a rare infection of neonates, elderly patients, and immunosuppressed patients, including those with HIV infection. It may also involve pregnant women. The organism, Listeria monocytogenes, is a Gram-positive coccobacillus that can replicate in macrophages. Bacteremia, with or without meningitis, is the usual clinical presentation. 915 Cutaneous lesions may be papular, pustular, or purpuric.
The epidermis shows mild spongiosis with lymphocyte exocytosis. 915 In pustular lesions subcorneal and intraepidermal neutrophils are present. The dermis contains a mixed inflammatory cell infiltrate with many macrophages. Gram-positive coccobacilli are present in macrophages, in adnexal structures, and lying free in the dermis or in epidermal inflammatory cells.
Cat-scratch disease is a self-limited illness characterized by the development, at the site of a scratch by a cat, of a papule or crusted nodule, which is followed approximately 2 weeks later by regional lymphadenitis.916.917.918. and 919. In 1–2% of patients severe systemic illness may develop: this may include an oculoglandular syndrome, pleurisy, encephalitis, osteomyelitis, 920 and splenomegaly. 921 Other cutaneous manifestations have included a plaque-like eruption, 922 erythema nodosum, urticaria, erythema marginatum, a Sweet’s syndrome-like eruption, 923 and purpura.919. and 924.
Small Gram-negative bacilli have been demonstrated in the skin916. and 921. and lymph nodes912.921.924.925.926. and 927. of most cases, using the Warthin–Starry silver stain or the Brown–Hopps modification of the Gram stain. The organism has been classified as Bartonella henselae. It is possible that the related bacillus Afipia felis may also be responsible (as originally reported), but there is a growing consensus that B. henselae is the predominant pathogen. B. clarridgeiae has been isolated from several cases. 922
The bacillus responsible for cat-scratch disease (B. henselae) has an etiological role in the causation of bacillary angiomatosis (epithelioid angiomatosis), a rare vascular proliferation seen in patients with the acquired immunodeficiency syndrome (see p. 911).928. and 929. Another eruptive vascular tumor, verruga peruana (see p. 912), is produced by another strain of Bartonella, B. bacilliformis.
Another species of Bartonella (B. quintana) is the agent of trench fever, a disease transmitted from human to human by the body louse. There is a high prevalence of this infection in homeless persons. 930
Although the lesions of verruga peruana usually resolve without antibiotics, these drugs are often used for the other Bartonella infections, particularly in immunosuppressed individuals. The quinolones and oral erythromycin are used in these circumstances. Azithromycin hastens resolution of adenopathy in cat-scratch disease. 931
The epidermal changes are variable and include acanthosis and ulceration. There is usually a zone of necrosis in the upper dermis, and this is surrounded by a mantle of macrophages and lymphocytes (Fig. 23.20). Neutrophils and eosinophils are often present around zones of necrosis. Multinucleate cells and well-defined granulomas may be present. Organisms are seen within macrophages and lying free, particularly in areas of necrosis and suppuration. 921 A rapid PCR-based detection of the causative organism can be carried out on fresh or paraffin-embedded samples. 933 The diagnosis can also be confirmed by immunohistochemistry using a commercially available monoclonal antibody. 934
Cat-scratch disease. There is a central zone of necrosis and a surrounding mantle of macrophages, lymphocytes, and some granulomas. (H & E)
Malakoplakia is a rare chronic inflammatory disease which occurs predominantly in the urogenital tract, especially the bladder. Fewer than 50 cases involving the skin and subcutaneous tissue have been reported.935.936.937.938. and 939. Nearly one-half of these patients were immunosuppressed; 940 occasionally this was due to the acquired immunodeficiency syndrome.941. and 942. Cutaneous involvement is usually in the vulva943 and perianal region,944. and 945. where yellow-pink indurated or polypoid masses have been described. Less common sites are the axilla, 946 forehead, 947 scalp, 948 and an injection site. 949
Although the pathogenesis of malakoplakia is uncertain, it appears to result from an acquired defect in the intracellular destruction of phagocytosed bacteria, usually Escherichia coli. 950 In the skin, Staph. aureus has also been isolated. 951
No deaths have been directly associated with cutaneous malakoplakia. 948 Treatment consists of a combination of surgery and antibiotic therapy. The highest cure rate is associated with the use of quinolones, and trimethoprim-sulfamethoxazole. 948 In the setting of organ transplantation, discontinuance or tapering of immunosuppressant therapy can also be considered. 948
Malakoplakia is a chronic inflammatory process characterized by sheets of closely packed macrophages containing PAS-positive diastase-resistant inclusions (von Hansemann cells). A variable proportion of these cells contain calcospherites with a homogeneous or target-like appearance – the Michaelis–Gutmann bodies. These stain with the von Kossa method. 951 There is also a scattering of lymphocytes and plasma cells. Immunostaining with polyclonal anti-Mycobacterium bovis antibodies, a method for detecting bacterial organisms in low concentration, has been successful in demonstrating bacteria in malakoplakia. 946
‘Sago palm’ disease, also known as Sepik granuloma, is a rare condition that appears to be restricted to the Sepik district of New Guinea. 952 It appears to follow injury by the sago palm (Metroxylon), but additional types of injury may also allow the entry of the causative organism, a Gram-positive bacillus. Affected individuals usually present with multiple cutaneous nodules on the extremities, but the face can also be involved. The organism has never been cultured.
There is a diffuse dermal infiltrate by large foamy histiocytes admixed with some plasma cells, lymphocytes, and fibroblasts. A syncytium of amorphous pink material extends between the histiocytes. It is PAS positive. The material contains numerous gray, round to oval dots, representing Gram-positive organisms surrounded by ground substance.
Chlamydiae are obligate intracellular organisms that share many features with bacteria, including a discrete cell wall. There are two morphologically distinct species, Chlamydia trachomatis and C. psittaci. The latter is responsible for psittacosis, a pneumonia derived from infected birds, whereas different serotypes of C. trachomatis are responsible for trachoma, urethritis, and lymphogranuloma venereum. The diagnosis can be confirmed by culture of the organism or by serology.
Psittacosis, caused by Chlamydia psittaci, usually presents as a pneumonic illness with various constitutional symptoms. Some patients develop a morbilliform rash or lesions resembling the rose spots of typhoid fever. Erythema nodosum, 953 erythema multiforme, erythema marginatum, 954 and disseminated intravascular coagulation955 with cutaneous manifestations have also been reported. The findings are not specific for psittacosis, either clinically or histopathologically.
Lymphogranuloma venereum (LGV), or lymphogranuloma inguinale, is a sexually transmitted disease caused by certain serovars/serotypes (L1, L2, and L3) of Chlamydia trachomatis.880. and 881. Worldwide in distribution, 956 it is most frequent in parts of Asia, Africa, and South America. Recent outbreaks, involving mainly the L2 strain have occurred in parts of Europe, and in the United States. 957 Most of these cases occurred in men having sex with men. 957 Clinically, three stages are recognized. 958 The initial lesion, which follows a few days or weeks after exposure to the organism, consists of a small papule, ulcer, or herpetiform vesicle on the penis, labia, or vaginal wall. Extragenital sites of involvement have been recorded. 959 The primary lesion is transient and often imperceptible. The secondary stage, which follows several weeks after the initial exposure, consists of regional lymphadenopathy. If the inguinal nodes are involved, enlarging buboes develop with draining sinuses. There may be constitutional symptoms at this stage. Erythema multiforme and erythema nodosum rarely develop. The tertiary stage, which is more common in women, encompasses the sequelae of the earlier inflammatory stages. 960 There may be rectal strictures, fistula formation, and rarely genital elephantiasis related to lymphedema.961. and 962. The duration of the disease may be prolonged in HIV-positive patients. 881
The organism can be isolated in a tissue culture system or in the yolk sac of eggs. 958 Serological methods are most commonly used to establish a diagnosis. They do not distinguish between the different serotypes of C. trachomatis. A microimmunofluorescence test can detect antichlamydial antibodies to a range of serological variants of C. trachomatis. 958 Various PCR-based tests have been developed in the past few years. DNA sequencing of the variable segments of the omp 1 gene (outer membrane protein gene) is used to type the serovars/serotypes. 957
Oral doxycycline is the treatment of choice. 957 Tetracycline is another alternative but the daily dose must be 2 g. Oral sulfadiazine and erythromycin have also been used.913. and 957.
The primary lesion is not commonly biopsied. Ulceration is usually present, with a dense underlying infiltrate of plasma cells and lymphocytes. 963 There is thickening of vessel walls and some endothelial swelling. Small sinus tracts may lead to the surface. A few epithelioid cell collections may be present. 963
The characteristic lesions occur in the lymph nodes in the second stage, with the development of stellate abscesses with a poorly formed palisade of epithelioid cells and histiocytes. Sinus formation also occurs. In later lesions there is variable fibrosis.
Direct immunofluorescence, using a fluorescein-labeled antibody to C. trachomatis, has been used to confirm the diagnosis. 964
Rickettsiae are small obligate intracellular bacteria which are transmitted in most instances by the bite of an arthropod. The various species of Rickettsia are endemic in different geographical locations, and this has influenced the naming of some of the infections.965.966. and 967.Table 23.1 lists the various rickettsial infections of humans, the corresponding etiological species, and the mode of transmission. The organism for scrub typhus has been reclassified from the genus Rickettsia to Orientia. 968
Rickettsiae usually produce an acute febrile illness accompanied by headache, myalgia, malaise, and morbidity. Rocky Mountain spotted fever has a mortality of approximately 5%.56.969.970.971. and 972. Its rash begins on the extremities and travels proximally to involve the trunk. 973 In the United States, children 5 to 9 years old have the highest incidence. 973 A specific diagnosis is usually made by serological methods, as the attempted isolation of rickettsiae in the laboratory is potentially highly hazardous for the laboratory staff. 974 Human granulocytic anaplasmosis (formerly human granulocytic ehrlichiosis), caused by the tick-borne rickettsia-like organism Anaplasma phagocytophilum, has some clinical similarities to Rocky Mountain spotted fever. 975 Having a similar vector, anaplasmosis is usually found in the United States in areas where Lyme disease is prevalent. 976 It has also been associated with Sweet’s syndrome. 977
Cutaneous lesions are present in most rickettsial infections, with the exception of Q fever. They are uncommon in African tick bite fever.967. and 978. This disease is now being seen in international travelers.979. and 980. Usually there is a macular or maculopapular rash; in rickettsialpox the rash may be papulovesicular.981. and 982. Rickettsialpox is endemic in New York City. 983 A more characteristic lesion is the eschar (tache noire), a crusted ulcer 1 cm or more in diameter that develops at the site of the arthropod bite. 984 It is thought to result not from the bite but from the inoculation of rickettsiae, which invade vascular endothelial cells. 985 The eschar in rickettsialpox needs to be distinguished from anthrax. 983 Eschars are characteristic of rickettsialpox,981. and 986. scrub typhus,987. and 988. boutonneuse fever,989. and 990. Queensland tick typhus, 991 and Siberian tick typhus.984. and 989. An eschar is not found in epidemic typhus or in murine typhus, and only rarely in Rocky Mountain spotted fever.965.984. and 989. Rickettsialpox has been reported in a patient with HIV infection. 992
Rickettsiae proliferate on the endothelium of small blood vessels, releasing cytokines which damage endothelial integrity. This leads to a cascade of events that may terminate in focal occlusive endangiitis. 993
Tetracyclines are the treatment of choice. 992 Doxycycline is the tetracycline most often used.
The pathological basis for the cutaneous lesions of rickettsial infections is a lymphocytic vasculitis. The eschars result from coagulative necrosis of the epidermis and underlying dermis.994. and 995. A crust overlies this region. Bordering the area of necrosis there is a lymphocytic vasculitis, with some fibrinoid necrosis of vessels and related dermal hemorrhage. 989 Arterioles, and not postcapillary venules are often involved. 967 Thrombosis of vessels is sometimes present. 989 There are often a few neutrophils in the inflammatory infiltrate. Numerous organisms can be demonstrated in vascular endothelium and vessel walls using fluorescein-labeled antisera to the appropriate rickettsial species.
The maculopapular lesions of Rocky Mountain spotted fever show a focal lymphocytic vasculitis with patchy fibrinoid necrosis of small vessels and extravasation of erythrocytes.996. and 997. Progression to a leukocytoclastic vasculitis is common.998. and 999. The most notable epidermal change is basal vacuolar degeneration with exocytosis of inflammatory cells. 998Rickettsia rickettsii can be demonstrated in endothelium and vascular walls using fluorescein-labeled antisera1000 or with immunoperoxidase techniques using routine sections. 998





