Atopic Dermatitis (Atopic Eczema): Introduction
|
Introduction
Atopic dermatitis (AD) is a chronically relapsing skin disease that occurs most commonly during early infancy and childhood. It is frequently associated with abnormalities in skin barrier function, allergen sensitization, and recurrent skin infections. There is no single distinguishing feature of AD or a diagnostic laboratory test. Thus, the diagnosis is based on the constellation of clinical findings listed in Table 14-1.1
Major Features |
Pruritus |
Rash on face and/or extensors in infants and young children |
Lichenification in flexural areas in older children |
Tendency toward chronic or chronically relapsing dermatitis |
Personal or family history of atopic disease: asthma, allergic rhinitis, atopic dermatitis |
Other Common Findings |
Dryness |
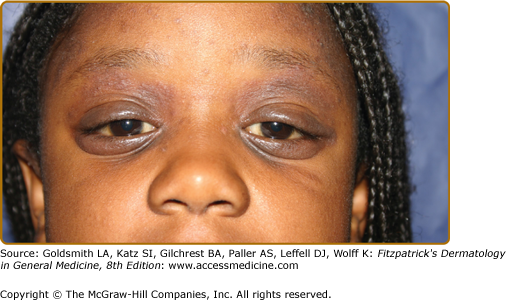
Dennie–Morgan folds (accentuated lines or grooves below the margin of the lower eyelid) |
Allergic shiners (darkening beneath the eyes) |
Facial pallor |
Pityriasis alba |
Keratosis pilaris |
Ichthyosis vulgaris |
Hyperlinearity of palms and soles |
White dermatographism (white line appears on skin within 1 minute of being stroked with blunt instrument) |
Conjunctivitis |
Keratoconus |
Anterior subcapsular cataracts |
Elevated serum immunoglobulin E |
Immediate skin test reactivity |
Epidemiology
Since the 1960s, there has been a more than threefold increase in the prevalence of AD.2 AD is a major public health problem worldwide, with a prevalence in children of 10–20% in the United States, Northern and Western Europe, urban Africa, Japan, Australia, and other industrialized countries.3 The prevalence of AD in adults is approximately 1–3%. Interestingly, the prevalence of AD is much lower in agricultural regions of countries such as China and in Eastern Europe, rural Africa, and Central Asia. However, the most recent data from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three study confirms that AD is a disease with high prevalence, affecting patients in both developed and developing countries.4 There is also a female preponderance for AD, with an overall female/male ratio of 1.3:1.0.
The basis for this increased prevalence of AD is not well understood. However, wide variations in prevalence have been observed within countries inhabited by similar ethnic groups, suggesting that environmental factors are critical in determining disease expression. Some of the potential risk factors that may be associated with the rise in atopic disease include small family size, increased income and education both in whites and blacks, migration from rural to urban environments, and increased use of antibiotics, that is, the so-called Western lifestyle.5,6 This has resulted in the “hygiene hypothesis” that allergic diseases might be prevented by “infection in early childhood transmitted by unhygienic contact with older siblings.”7 Given the increase in autoimmune diseases such as diabetes, abnormalities in T regulatory cells have also been implicated.
Etiology and Pathogenesis
AD is a highly pruritic inflammatory skin disease that results from complex interactions between genetic susceptibility genes resulting in a defective skin barrier, defects in the innate immune system, and heightened immunologic responses to allergens and microbial antigens.8
AD is associated with a marked decrease in skin barrier function due to the downregulation of cornified envelope genes (filaggrin and loricrin), reduced ceramide levels, increased levels of endogenous proteolytic enzymes, and enhanced transepidermal water loss.9,10 Addition of soap and detergents to the skin raises its pH, thereby increasing activity of endogenous proteases, leading to further breakdown of epidermal barrier function. The epidermal barrier may also be damaged by exposure to exogenous proteases from house dust mites and Staphylococcus aureus (S. aureus). This is worsened by the lack of certain endogenous protease inhibitors in atopic skin. These epidermal changes likely contribute to increased allergen absorption into the skin and microbial colonization. Because epicutaneous, as compared to systemic or airway, sensitization to allergen results in higher level allergic immune responses, decreased skin barrier function could act as a site for allergen sensitization and predispose such children to the development of food allergy and respiratory allergy.11
Clinically unaffected skin of AD patients manifests mild epidermal hyperplasia and a sparse perivascular T cell infiltrate.12 Acute eczematous skin lesions are characterized by marked intercellular edema (spongiosis) of the epidermis. Dendritic antigen-presenting cells [e.g., Langerhans cells (LCs), macrophages] in lesional and, to a lesser extent, in nonlesional skin of AD exhibit surface-bound immunoglobulin E (IgE) molecules. A sparse epidermal infiltrate consisting primarily of T lymphocytes is also frequently observed.
In the dermis of the acute lesion, there is an influx of T cells with occasional monocyte-macrophages. The lymphocytic infiltrate consists predominantly of activated memory T cells bearing CD3, CD4, and CD45 RO (suggesting previous encounter with antigen). Eosinophils are rarely present in acute AD. Mast cells are found in normal numbers in different stages of degranulation.
Chronic lichenified lesions are characterized by a hyperplastic epidermis with elongation of the rete ridges, prominent hyperkeratosis, and minimal spongiosis. There is an increased number of IgE-bearing LCs in the epidermis, and macrophages dominate the dermal mononuclear cell infiltrate. Mast cells are increased in number but are generally fully granulated. Neutrophils are absent in AD skin lesions even in the setting of increased S. aureus colonization and infection. Increased numbers of eosinophils are observed in chronic AD skin lesions. These eosinophils undergo cytolysis with release of granule protein contents into the upper dermis of lesional skin. Eosinophil-derived extracellular major basic protein can be detected in a fibrillar pattern associated with the distribution of elastic fibers throughout the upper dermis. Eosinophils are thought to contribute to allergic inflammation by the secretion of cytokines and mediators that augment allergic inflammation and induce tissue injury in AD through the production of reactive oxygen intermediates and release of toxic granule proteins.
Atopic skin inflammation is orchestrated by the local expression of proinflammatory cytokines and chemokines.12 Cytokines such as tumor necrosis factor-α (TNF-α) and interleukin 1 (IL-1) from resident cells [keratinocytes, mast cells, dendritic cells (DCs)] bind to receptors on the vascular endothelium, activating cellular signaling pathways, which leads to the induction of vascular endothelial cell adhesion molecules. These events initiate the process of tethering, activation, and adhesion to vascular endothelium followed by extravasation of inflammatory cells into the skin. Once inflammatory cells have infiltrated into the skin, they respond to chemotactic gradients established by chemokines that emanate from sites of injury or infection.
Acute AD is associated with the production of T helper 2 type (Th2) cytokines, notably IL-4 and IL-13,13 which mediate immunoglobulin isotype switching to IgE synthesis and upregulate expression of adhesion molecules on endothelial cells. The important role that Th2 cytokines play in the skin’s inflammatory response is supported by the observation that transgenic mice genetically engineered to overexpress IL-4 in their skin develop inflammatory pruritic skin lesions similar to AD, suggesting that local skin expression of Th2 cytokines plays a critical role in AD. There has also been considerable interest in IL-31, which is a novel Th2 cytokine that induces severe pruritus and dermatitis in experimental animals. IL-31 has also been found to be increased in AD skin and serum levels of IL-31 correlate with severity of skin disease.14
In chronic AD, there is an increase in the production of IL-5, which is involved in eosinophil development and survival. Increased production of granulocyte macrophage colony-stimulating factor in AD inhibits apoptosis of monocytes, thereby contributing to the persistence of AD.15 The maintenance of chronic AD also involves production of the Th1-like cytokines IL-12 and IL-18, as well as several remodeling-associated cytokines, including IL-11 and transforming growth factor-1.16
The skin-specific chemokine, cutaneous T cell-attracting chemokine [CTACK; CC chemokine ligand 27 (CCL27)], is highly upregulated in AD and preferentially attracts skin homing cutaneous lymphoid antigen (CLA)+ CC chemokine receptor 10+ (CCR10+) T cells into the skin.17 CCR4 expressed on skin homing CLA+ T cells can also bind to CCL17 on the vascular endothelium of cutaneous venules. Selective recruitment of CCR4-expressing Th2 cells is mediated by macrophage-derived chemokine and thymus and activation-regulated cytokine, both of which are increased in AD. Severity of AD has been linked to the magnitude of thymus and activation-regulated cytokine levels. In addition, chemokines such as fractalkine, interferon (IFN)-γ-inducible protein 10, and monokine induced by IFN-γ are strongly upregulated in keratinocytes and result in Th1-cell migration toward epidermis, particularly in chronic AD. Increased expression of the CC chemokines, macrophage chemoattractant protein-4, eotaxin, and RANTES (regulated on activation, normal T cell expressed and secreted) contribute to infiltration of macrophages, eosinophils, and T cells into both acute and chronic AD skin lesions.
DCs play an important role in detecting environmental allergens or pathogens via pattern recognition receptors such as toll-like receptors (TLR). AD skin contains two types of high-affinity, IgE receptor-bearing (FcϵR) myeloid DCs: (1) LCs and (2) inflammatory dendritic epidermal cells (IDECs). IgE-bearing LCs appear to play an important role in cutaneous allergen presentation to IL-4-producing Th2 cells.18 In this regard, IgE-bearing LCs from AD skin lesions, but not LCs that lack surface IgE, are capable of presenting allergens to T cells. These results suggest that cell-bound IgE on LCs facilitates capture and internalization of allergens into LCs before their processing and antigen presentation to T cells. IgE-bearing LCs that have captured allergen likely activate memory Th2 cells in atopic skin, but they may also migrate to the lymph nodes to stimulate naïve T cells there to further expand the pool of systemic Th2 cells. Stimulation of FcϵRI on the surface of LCs by allergens induces the release of chemotactic signals and recruitment of precursor cells of IDECs and T cells in vitro. Stimulation of FcϵRI on IDECs leads to the release of proinflammatory signals, which contribute to amplification of the allergic immune response.
In contrast to other inflammatory skin diseases, such as allergic contact dermatitis or psoriasis vulgaris, very low numbers of plasmacytoid DCs (pDCs), which play an important role in host defense against viral infections, can be detected within the AD skin lesion.19 pDCs in the peripheral blood of patients with AD have been shown to bear the trimeric variant of FcϵRI on their cell surface, which is occupied by IgE molecules. The modified immune function of pDCs of patients with AD after FcϵRI-mediated allergen stimulation might contribute to a local deficiency of type I IFNs, thereby contributing to increased susceptibility of AD patients toward viral skin infections such as eczema herpeticum.20
Skin homing memory T cells play an important role in the pathogenesis of AD, particularly during the acute phase of illness. This concept is supported by the observation that primary T-cell immunodeficiency disorders are frequently associated with eczematous skin lesions that clear after successful bone marrow transplantation.21 Furthermore, in animal models of AD, the eczematous rash does not occur in the absence of T cells. In addition, treatment with topical calcineurin inhibitors (TCIs), which target activated T cells, significantly reduces the clinical skin rash of AD.22
Several studies have demonstrated the presence of Th2-like T cells in acute AD that produce cytokines that enhance allergic skin inflammation. During the chronic phase of AD, there is a switch to Th1-like cells that primarily produce IFN-γ. These Th1-like cells induce the activation and apoptosis of keratinocytes.23 Recently, T regulatory (Treg) cells have been described as a further subtype of T cells that have immunosuppressive function and cytokine profiles distinct from both Th1 and Th2 cells.24 Treg cells are able to inhibit the development of both Th1 and Th2 responses. Mutations in a nuclear factor expressed in Treg cells, FoxP3, result in IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome characterized by elevated serum IgE, food allergy, and dermatitis that may be eczematous or psoriasiform. A deficiency of resident Treg cells has also been reported in AD skin.25 Interestingly, staphylococcal superantigens subvert Treg cell function and may thereby augment skin inflammation.26
There has also been considerable interest in the role of Th17 cells in the immunopathogenesis of AD.27 These cells produce inflammatory cytokines such as IL-17 and are thought to play a role in host defense by inducing keratinocytes to produce antimicrobial peptides as well as promote neutrophil chemotaxis. Th17 cells are increased in the skin lesions of autoimmune diseases, such as psoriasis, where they may promote inflammatory responses, including neutrophil infiltration but also reduce skin infection.28 Compared to psoriasis, AD skin lesions have significantly fewer T cells expressing IL-17, but increased numbers of IL-4+ cells.29 Furthermore, it has been found that the Th2 cytokines, IL-4 and IL-13, inhibit IL-17 induced generation of antimicrobial peptides.30 Interestingly, an independent increase of IL-22 expressing cells, originally thought to be produced by Th17 cells, can be found in AD skin and it has been suggested that these may contribute to epidermal hyperplasia.31
Keratinocytes play a critical role in the augmentation of atopic skin inflammation. AD keratinocytes secrete a unique profile of chemokines and cytokines after exposure to proinflammatory cytokines. This includes high levels of RANTES after stimulation with TNF-α and IFN-γ.32 They are also an important source of thymic stromal lymphopoietin (TSLP), which activates DCs to prime naïve T cells to produce IL-4 and IL-13 (i.e., promotes Th2 cell differentiation).33 The importance of TSLP in AD pathogenesis is supported by the observation that mice genetically manipulated to overexpress TSLP in the skin develop AD-like skin inflammation. Skin-derived TSLP is also thought to trigger the development of asthma.34,35
Keratinocytes are critical to the skin’s innate immune responses, expressing Toll-like receptors, producing proinflammatory cytokines and antimicrobial peptides (such as human β defensins and cathelicidins) in response to tissue injury or invading microbes.36 Several studies have now demonstrated that AD keratinocytes produce reduced amounts of antimicrobial peptides and this may predispose such individuals to skin colonization and infection with S. aureus, viruses, and fungi. However, this defect appears to be acquired as the result of Th2-cytokine (IL-4, IL-10, and IL-13) mediated inhibition of TNF-α and IFN-γ-induced antimicrobial peptide generation.
AD is a complex disease that is familially transmitted with a strong maternal influence.37 Genome-wide linkage studies of families with AD have implicated chromosomal regions that overlap with other inflammatory skin diseases such as psoriasis. Together with candidate gene studies, these have provided interesting insights into the pathogenesis of AD. Although many genes are likely to be involved in the development of AD, there has been particular interest in the potential role of skin barrier/epidermal differentiation genes and immune response/host defense genes.
Loss-of-function mutations in FLG, which encodes the epidermal barrier protein, filaggrin, have been demonstrated to be a major predisposing factor for AD,38 as well as ichthyosis vulgaris, a common keratinizing disorder associated with AD (Figs. 14-1 and 14-2). Patients with filaggrin null mutations often have early onset, severe eczema, high level allergen sensitization, and develop asthma later in childhood. Of note, the filaggrin gene is found on chromosome 1q21 that contains genes (including loricrin and S100 calcium-binding proteins) in the epidermal differentiation complex, known to be expressed during terminal differentiation of the epidermis. DNA microarray analyses have demonstrated upregulation of S100 calcium-binding proteins and downregulation of loricrin and filaggrin in AD. Candidate gene approaches have also implicated variants in the SPINK5 gene, which is expressed in the uppermost epidermis where its product, LEKT1, inhibits two serine proteases involved in desquamation and inflammation (stratum corneum tryptic enzyme and stratum corneum chymotryptic enzyme). Stratum corneum tryptic enzyme and stratum corneum tryptic enzyme expression is increased in AD, suggesting that an imbalance of protease versus protease inhibitor activity may contribute to atopic skin inflammation.9
These observations establish a key role for impaired skin barrier function in the pathogenesis of AD, as impaired skin barrier formation allows increased transepidermal water loss and, importantly, increased entry of allergens, antigens, and chemicals from the environment resulting in skin inflammatory responses. It is important to note that these filaggrin mutations, and likely other mutations affecting the skin barrier, can occur in clinically normal individuals, and in patients with ichthyosis vulgaris without clinical evidence of skin inflammation. The majority of patients with AD outgrow their inflammatory skin disease by adolescence. Thus, AD is a complex trait that involves interactions between multiple gene products requiring environmental factors and the immune response to result in the final clinical phenotype. Chromosome 5q31-33 contains a clustered family of functionally related cytokine genes—IL-3, IL-4, IL-5, IL-13, and granulocyte macrophage colony-stimulating factor—which are expressed by Th2 cells. A case control comparison has suggested a genotypic association between the T allele of the 590C/T polymorphism of the IL-4 gene promoter region with AD. Because the T allele is associated with increased IL-4 gene promoter activity when compared to the C allele, this suggests that genetic differences in transcriptional activity of the IL-4 gene influence AD predisposition. In addition, an association of AD with a gain-of-function mutation in the α subunit of the IL-4 receptor has been reported, providing further support of the concept that IL-4 gene expression plays a role in AD. Functional mutations in the promoter region of the CC chemokines, RANTES, and eotaxin, as well as variants in IL-13, the α subunit of the high affinity cell surface receptor for IgE (FcϵR1) found on basophils and mast cells suggest an overlapping of genetic basis with other atopic diseases.
Recent studies demonstrating a significant association between TSLP gene polymorphisms and AD provide further support for the importance of Th2 polarization in this disease.37 The involvement of T cell γ interferon and IL-18 genes support the role of CD4+ T cells and dysregulation of Th1 genes in the pathophysiology of AD. As well, reports of AD association with polymorphisms of the NOD1 gene, which encodes cytosolic pathogen recognition receptor and toll-like receptors, suggest an important role for host defense genes in the pathogenesis of AD. The reader is referred to Chapter 10 and reference 35 for a detailed discussion of the genetics of AD.
Pruritus is a prominent feature of AD, manifested as cutaneous hyperreactivity and scratching following exposure to allergens, changes in humidity, excessive sweating, and low concentrations of irritants. Control of pruritus is important because mechanical injury from scratching can induce proinflammatory cytokine and chemokine release, leading to a vicious scratch–itch cycle perpetuating the AD skin rash. The mechanisms of pruritus in AD are poorly understood. Allergen-induced release of histamine from skin mast cells is not an exclusive cause of pruritus in AD, because H1 antihistamines are not effective in controlling the itch of AD.39 However, recent studies demonstrating a potential role for H4 receptors in skin pathobiology suggests that histamine may play a contributory role.40 However, the observation that treatment with topical corticosteroids and calcineurin inhibitors is effective at reducing pruritus suggests that the inflammatory cells play an important role in pruritus.41,42 Molecules that have been implicated in pruritus include T-cell-derived cytokines such as IL-31, stress-induced neuropeptides, and proteases which can act on protease-activated receptors, eicosanoids, and eosinophil-derived proteins.43,44 The reader is referred to Chapter 103 for a detailed discussion of the pathophysiology of pruritus.
Clinical Findings
The diagnosis of AD is based on the constellation of clinical features summarized in Table 14-1. AD typically begins during infancy. Approximately 50% of patients develop this illness by the first year of life and an additional 30% between the ages of 1–5 years. Between 50–80% of patients with AD develop allergic rhinitis or asthma later in childhood. Many of these patients outgrow their AD as they are developing respiratory allergy.
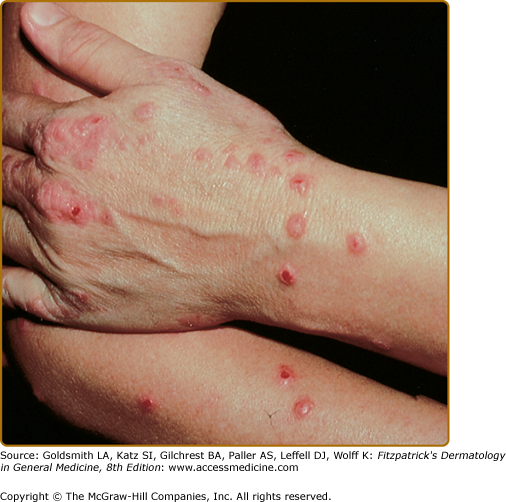
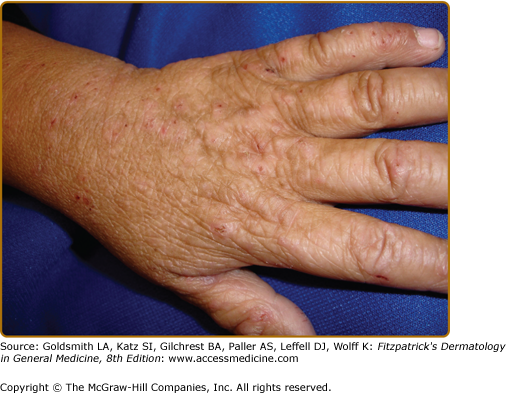
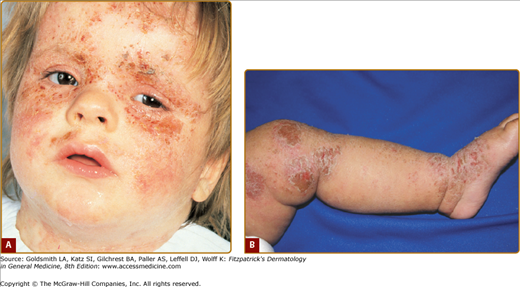
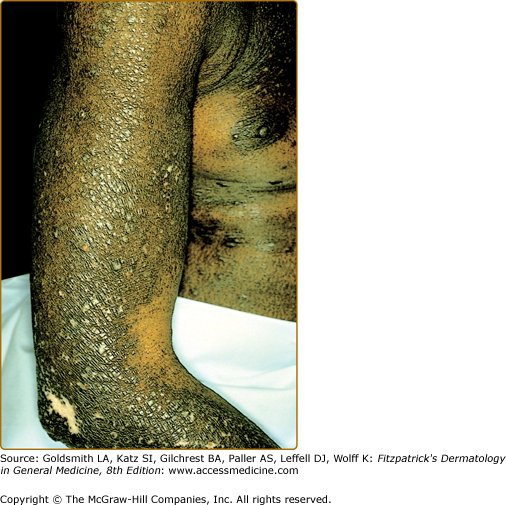
Intense pruritus and cutaneous reactivity are cardinal features of AD. Pruritus may be intermittent throughout the day but is usually worse in the early evening and night. Its consequences are scratching, prurigo papules (Fig. 14-3), lichenification (Fig. 14-4), and eczematous skin lesions. Acute skin lesions are characterized by intensely pruritic, erythematous papules associated with excoriation, vesicles over erythematous skin, and serous exudate (Fig. 14-5). Subacute dermatitis is characterized by erythematous, excoriated, scaling papules (Fig. 14-6). Chronic AD is characterized by (1) thickened plaques of skin, (2) accentuated skin markings (lichenification), and (3) fibrotic papules (prurigo nodularis; Fig. 14-7). In chronic AD, all three stages of skin reactions frequently coexist in the same individual. At all stages of AD, patients usually have dry, lackluster skin.
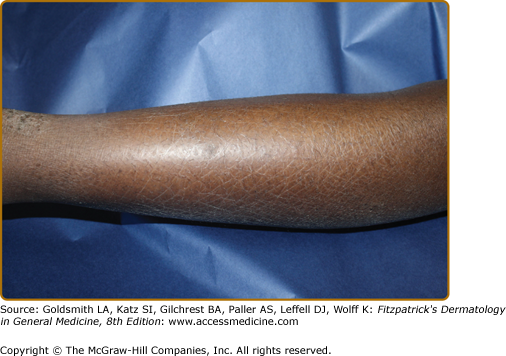
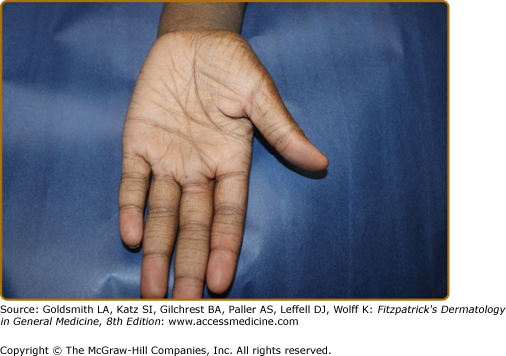
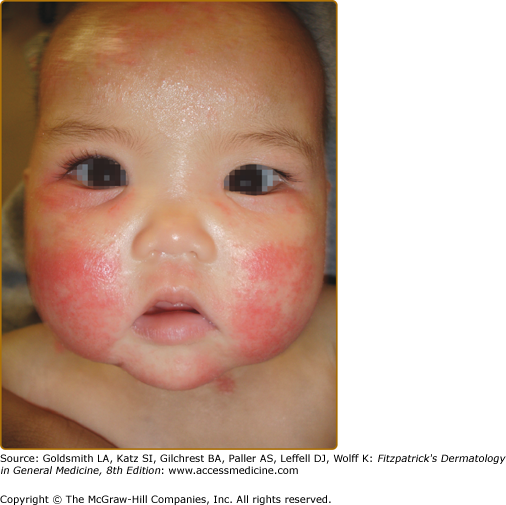
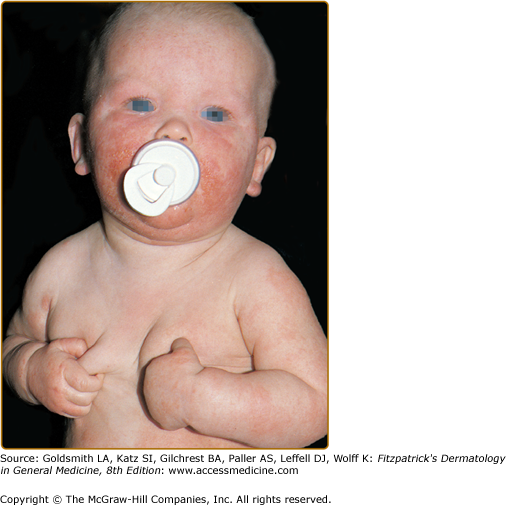
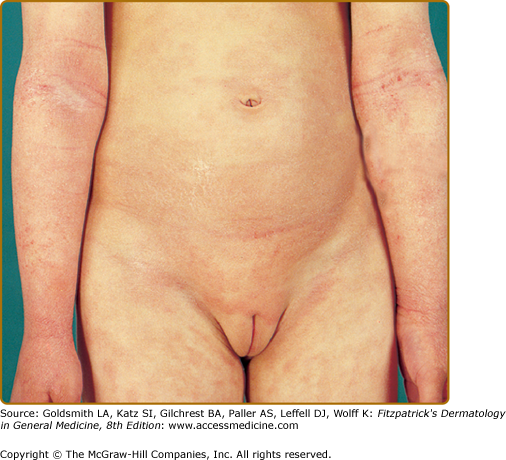
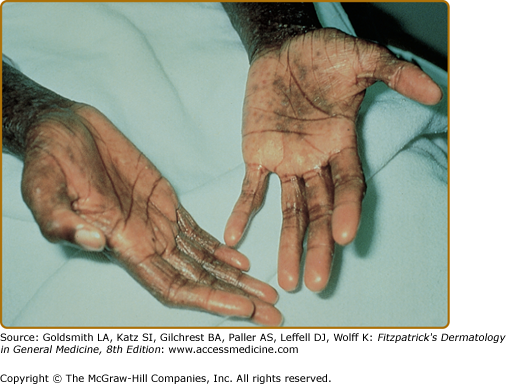
The distribution and skin reaction pattern vary according to the patient’s age and disease activity. During infancy, the AD is generally more acute and primarily involves the face (Fig. 14-8), scalp, and the extensor surfaces of the extremities (Fig. 14-9). The diaper area is usually spared. In older children, and in those who have long-standing skin disease, the patient develops the chronic form of AD with lichenification and localization of the rash to the flexural folds of the extremities (Fig. 14-10). AD often subsides as the patient grows older, leaving an adult with skin that is prone to itching and inflammation when exposed to exogenous irritants. Chronic hand eczema may be the primary manifestation of many adults with AD (Fig. 14-11). Other associated features of AD are listed in Table 14-1.
Laboratory testing is not needed in the routine evaluation and treatment of uncomplicated AD. Serum IgE levels are elevated in approximately 70–80% of AD patients. This is associated with sensitization against inhalant and food allergens and/or concomitant allergic rhinitis and asthma.8 In contrast, 20–30% of AD patients have normal serum IgE levels. This subtype of AD has a lack of IgE sensitization against inhalant or food allergens. However, some of these patients may possess IgE sensitization against microbial antigens such as S. aureus toxins, and Candida albicans or Malassezia sympodialis can be detected. As well, some of these patients show positive reactions using the atopy patch test despite negative immediate skin tests.
The majority of patients with AD also have peripheral blood eosinophilia. Patients with AD have increased spontaneous histamine release from basophils. These findings likely reflect a systemic Th2 immune response in AD especially those patients who have elevated serum IgE levels. Importantly, the peripheral blood skin homing CLA+ T cells in AD expressing either CD4 or CD8 spontaneously secrete IL-5 and IL-13, which functionally prolong eosinophil survival and induce IgE synthesis.
Diagnosis and Differential Diagnosis
Table 14-1 lists the clinical features of AD. Of the major features, pruritus and chronic or remitting eczematous dermatitis with typical morphology and distribution are essential for diagnosis. Other features, including allergy or elevated IgE, are variable, and some of the “associated features” in the table may not be useful discriminators of individuals with AD from the unaffected general population. Various diagnostic criteria have been proposed to assist with clinical diagnosis, definition of patients for clinical studies, and epidemiologic population studies.45 A refined list of diagnostic criteria suitable for epidemiologic studies has been derived and validated by workers in the United Kingdom.46
Box 14-1 lists a number of inflammatory skin diseases, immunodeficiencies, skin malignancies, genetic disorders, infectious diseases, and infestations that share symptoms and signs with AD. These should be considered and ruled out before a diagnosis of AD is made. Infants presenting in the first year of life with failure to thrive, diarrhea, a generalized scaling erythematous rash, and recurrent cutaneous and/or systemic infections should be evaluated for severe combined immunodeficiency syndrome. Wiskott–Aldrich syndrome is an X-linked recessive disorder characterized by cutaneous findings almost indistinguishable from AD (see Chapter 143). It is associated with thrombocytopenia, variable abnormalities in humoral and cellular immunity, and recurrent severe bacterial infections. The hyper-IgE syndrome is characterized by elevated serum IgE levels, defective T-cell function, recurrent deep-seated bacterial infections, including cutaneous abscesses due to S. aureus and/or pruritic skin disease due to S. aureus pustulosis, or by recalcitrant dermatophytosis. A papulopustular eruption of the face and scalp may be seen in early life. Although S. aureus is an important pathogen in this disorder, infection with other bacteria, viruses, and fungi may occur, particularly when patients are on chronic antistaphylococcal antibiotic prophylaxis. Hyper-IgE is most commonly an autosomal dominant disorder due to mutations in STAT3, which also features pneumonia with pneumatocele formation, dental anomalies with retained primary teeth, bone fractures, and osteopenia. Autosomal recessive forms of hyper-IgE syndrome show severe eosinophilia, recurrent viral and bacterial infections, an increased risk of autoimmune disease, and serious neurologic manifestations, but not the pneumatoceles and dental or skeletal defects. To date, deficiencies of Tyk2 and dedicator of cytokinesis 8 protein (DOCK8) deficiency have been found, leading to a global defect in T-cell activation.47
MOST LIKELY |
Contact dermatitis (allergic and irritant) Seborrheic dermatitis Scabies Psoriasis Ichthyosis vulgaris Keratosis pilaris Dermatophytosis |
CONSIDER |
Asteatotic eczema Lichen simplex chronicus Nummular dermatitis Juvenile palmar–plantar dermatosis Impetigo Drug eruptions Perioral dermatitis Pityriasis alba Photosensitivity disorders (hydroa vacciniforme; polymorphous light eruption, porphyrias) Molluscum dermatitis |
LESS COMMON/RARE DISORDERS PREDOMINANT IN ADOLESCENTS AND ADULTS |
Cutaneous T-cell lymphoma (mycosis fungoides or Sézary syndrome) Human immunodeficiency virus-associated dermatoses Lupus erythematosus Dermatomyositis Graft-versus-host disease Pemphigus foliaceus Dermatitis herpetiformis Photosensitivity disorders (hydroa vacciniforme, polymorphous light eruption, porphyrias) |
LESS COMMON/RARE DISORDERS PREDOMINANT IN INFANTS/CHILDREN |
Metabolic/Nutritional |
Phenylketonuria Prolidase deficiency Multiple carboxylase deficiency Zinc deficiency (acrodermatitis enteropathica; prematurity; deficient breast milk zinc; cystic fibrosis) Others: biotin, essential fatty acids, organic acidurias |
Primary Immunodeficiency Disorders |
Severe combined immunodeficiency disorder DiGeorge syndrome Hypogammaglobulinemia Agammaglobulinemia Wiskott–Aldrich syndrome Ataxia-telangiectasia Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome Hyperimmunoglobulin E syndrome (autosomal dominant and recessive forms) Chronic mucocutaneous candidiasis Omenn syndrome |
Other Genetic Syndromes Netherton syndrome |
Inflammatory, Autoimmune Disorders Eosinophilic gastroenteritis Gluten-sensitive enteropathy Neonatal lupus erythematosus |
Proliferative Disorders |
Langerhans cell histiocytosis |
It is important to recognize that an adult who presents with an eczematous dermatitis with no history of childhood eczema, respiratory allergy, or atopic family history may have allergic contact dermatitis. A contact allergen should be considered in any patient whose AD does not respond to appropriate therapy. Of note, contact allergy to topical glucocorticoids and TCIs has been reported in patients with chronic dermatitis. In addition, cutaneous T-cell lymphoma must be ruled out in any adult presenting with chronic dermatitis poorly responsive to topical glucocorticoid therapy. Ideally, biopsies should be obtained from three separate sites, because the histology may show spongiosis and cellular infiltrate similar to AD. Eczematous dermatitis has been also reported with human immunodeficiency virus as well as with a variety of infestations such as scabies. Other conditions that can be confused with AD include psoriasis, ichthyoses, and seborrheic dermatitis.