Comprehensive multispecialty ‘eczema clinics’ are beginning to test the utility of intensive education as a distinct component of long-term AD management. This model, with its longer appointments, focused educational curricula, patient support networks, and the ability to elicit patient/family feedback, parallels strategies shown to be effective for managing asthma, diabetes, and other chronic diseases. Supporters hope to empower patients and caregivers and improve both clinical and quality of life outcomes. Long-term comparative evaluation is required to examine the cost-effectiveness and suitability of these educational programs. Other learning modalities beyond in-clinic disease education may be useful to assist with disease management, including hand-outs, internet-based written material and instructional videos (e.g., www.eczemacenter.org; www.nationaleczema.org; www.eczema.org).
Atopic dermatitis
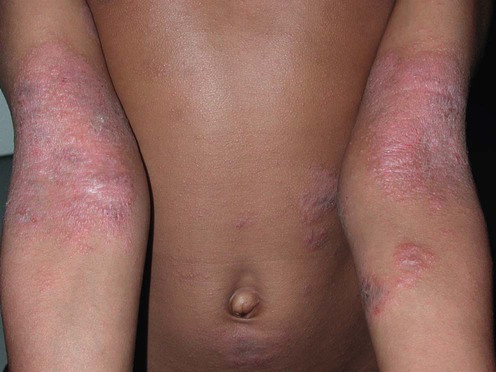
Management strategy
Interventional education
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Plastic Surgery Key
Fastest Plastic Surgery & Dermatology Insight Engine
