4 Anesthesia for upper extremity surgery
Synopsis
 Optimal perioperative anesthetic outcomes are achieved by a thorough understanding of anatomy, pharmacology, techniques, and potential complications.
Optimal perioperative anesthetic outcomes are achieved by a thorough understanding of anatomy, pharmacology, techniques, and potential complications.
 Local anesthetics (LA) make regional anesthesia possible by preventing the propagation of nerve conduction and by inhibiting or relieving pain.
Local anesthetics (LA) make regional anesthesia possible by preventing the propagation of nerve conduction and by inhibiting or relieving pain.
 Ultrasound guidance in the use of regional anesthesia has decreased the need for high volumes of anesthetic.
Ultrasound guidance in the use of regional anesthesia has decreased the need for high volumes of anesthetic.
Anatomy
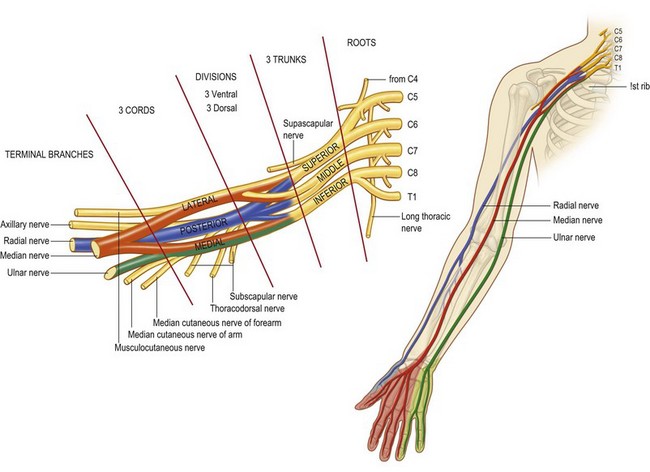
The brachial plexus arises from the ventral rami of nerves C5–8 and T1, with variable contributions from C4 and T2 (Fig. 4.1). These rami unite and diverge forming the roots, trunks, divisions, cords, and terminal nerves of the brachial plexus. C5 and C6 roots form the superior trunk, C7 becomes the middle trunk, and C8 and T1 form the inferior trunk between the anterior and middle scalene muscles. The three trunks then divide into anterior and posterior divisions, coursing over the first rib and lateral to the subclavian artery. The divisions then reunite to form cords. The anterior divisions of the superior and middle trunk form the lateral cord, while the anterior division of the inferior trunk forms the medial cord. The posterior divisions of all three trunks form the posterior cord. The cords are named according to their anatomic relationship with the axillary artery. The cords then divide once again to become the terminal branches of the brachial plexus. The lateral cord gives rise to the musculocutaneous nerve and contributes to the median nerve. The medial cord also contributes to the median nerve and gives rise to the ulnar nerve and the medial brachial and antebrachial cutaneous nerves. The posterior cord becomes the axillary and radial nerves.1
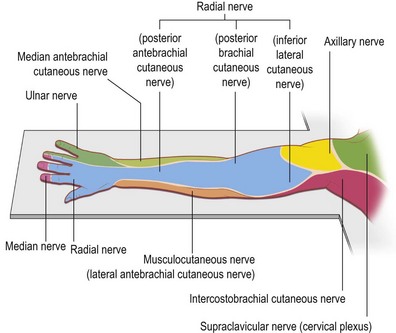
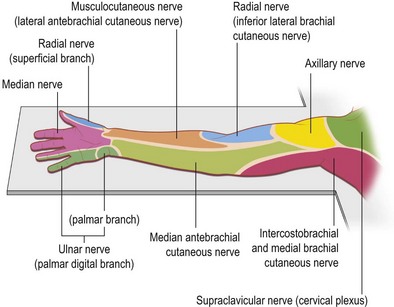
Knowledge of brachial plexus anatomy and the dermatomes supplied (Figs 4.2, 4.3), enables selective regional anesthesia.
Perineurial environment
The axillary sheath is the connective tissue surrounding the neurovascular structures of the brachial plexus. It originates as a continuation of the prevertebral fascia and joins the fascia of the biceps and brachialis muscles distally. This connective tissue extends inward, forming septa between components of the plexus and creating fascial compartments for each nerve.2 Controversy exists regarding the ability of the septae to limit the spread of local anesthetics within the sheath. Some investigators report that these fascial compartments limit the circumferential spread of local anesthetics and that injected solutions spread longitudinally up and down the nerve and remain compartmentalized. This concept provides a rational explanation for the occurrence of a rapid and profound block of one nerve, yet partial or absent block in other nerves during brachial plexus blockade.3 Other investigators propose that these septa are incomplete and form small bubble-like pockets when solution is injected. They found that single injections of dye solutions into the axillary sheath resulted in immediate staining of median, radial, and ulnar nerves, despite the presence of septa. These data demonstrate that there are connections between compartments within the sheath, and may explain why single injection techniques have success rates comparable with multiple injection techniques during blockade of the brachial plexus.4
Microneuroanatomy
Peripheral nerves are composed of fascicles of individual nerve fibers surrounded by endoneurium. Groups of fascicles are contained within the epineurium. As the nerve travels away from the spinal cord, fascicle numbers increase, while fascicle size decreases.5 The nerve roots contain large fascicles, demonstrating a monofascicular or oligofascicular pattern, while a multifascicular pattern is found more distally.6,7 While the amount of neural tissue remains constant, the amount of non-neural connective tissue increases from proximal to distal. The ratio of neural to non-neural tissue changes from 1 : 1 in the proximal plexus to 1 : 2 in the more distal plexus.2,7 The presence of non-neural tissue may explain why injections within the epineurium rarely result in neural injury.6
Sonoanatomy
The shape and echogenicity of a nerve determine its ultrasound appearance. Structures that strongly reflect ultrasound waves generate large signal intensities and appear white or hyperechoic. In contrast, hypoechoic structures weakly reflect ultrasound waves and appear darker.8 Peripheral nerves show a mixture of hypoechoic and hyperechoic structures constituting a typical “honeycomb” structure.9 Hypoechoic structures seen with ultrasound correspond to neural tissue, while hyperechoic areas correlate with connective tissue.10 Ultrasound imaging of the proximal brachial plexus usually shows hypoechoic structures reflecting an oligofascicular pattern. Distal brachial plexus structures display a more hyperechoic, honeycomb appearance, reflecting a multifascicular pattern.7
Pharmacology of local anesthetics
Local anesthetics (LA) make regional anesthesia possible by preventing the propagation of nerve conduction and inhibiting or relieving pain.11 LA are primarily weak bases that attach to sites of the sodium channel in nerves and prevent the movement of the sodium ion through the nerve pores, which temporarily halts nerve conduction.12
Toxicity
Local anesthetic toxicity has been a concern since its first use in nerve blockade. Regardless of which local anesthetic is injected, traditional methods have required large volumes for successful regional anesthesia such as with brachial plexus blockade or Bier blocks. Frequent aspiration and incremental dosing are imperative, as is communication with the patient to detect early signs of potential intravenous injection before progression to signs and symptoms of toxicity. Factors such as drug dose, rate of absorption, biotransformation, and elimination of the drug from the circulation are determinants of the plasma concentration of local anesthetics. Fortunately, one of the benefits of ultrasound guidance in the use of regional anesthesia has been the decreased need for large volumes.13
Physicians must be prepared with monitors, emergency drugs, and airway supplies to facilitate treatment of LA related toxicity. Toxicity related to LA can include but is not limited to oxygen desaturation, hypotension, bradycardia, and seizures. The extent of toxicity is determined by the specific drug’s intrinsic properties as well as the plasma level of the LA. The safety of LA is an essential aspect of regional anesthesia and is dependent on the skill of the physician, placement of the needle, the drug utilized, and patient health. All of these factors must be considered when determining the appropriate procedure and LA dose.14
Vasoconstrictors
With the addition of vasoconstrictors such as epinephrine or phenylephrine to the LA anesthetic solution, the systemic absorption rate of an LA can be decreased.14 The dose of bupivacaine increases from 3.5 to 4.0 mg/kg. This action of epinephrine is more significant with lidocaine as the upper limit increases from 3.0 to 7.0 mg/kg. Vasoconstrictors allow the physician to identify an intravascular injection sooner rather than later, due to the development of tachycardia with an intravascular injection.15 The block can be halted immediately, possibly preventing a more serious intravascular complication. Vasoconstrictors result in decreased plasma uptake and increased duration of local anesthetic effect.16
LA selection
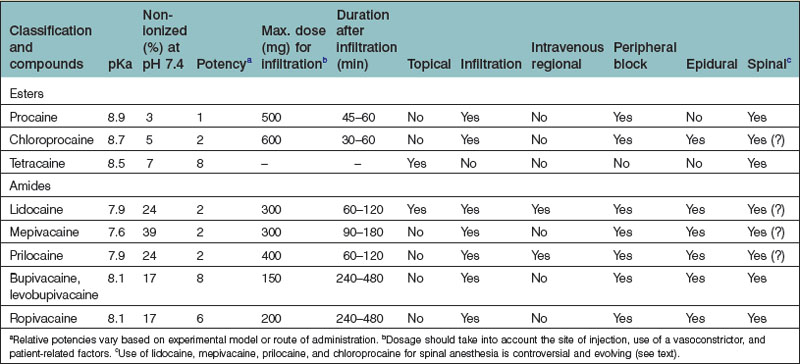
The choice of a LA depends on toxicity (as discussed previously), duration of effect, and time to onset (Table 4.1).
Table 4.1 Commonly used agents in upper extremity regional anesthesia
| Lidocaine | Most widely used LA |
| Prototype amide | |
| Can be used in almost any peripheral block | |
| 1.5% or 2% with or without epinephrine is most commonly used for surgical anesthesia | |
| Mepivacaine | Intermediate duration |
| Similar to lidocaine | |
| Less vasodilation | |
| Ineffective as topical agent | |
| 1.5% mepivacaine is the most commonly used agent in regional anesthesia | |
| Bupivacaine | One of the most commonly used LA in regional and infiltration anesthesia |
| Long-acting | |
| High quality sensory anesthesia relative to motor blockade | |
| Most commonly used for epidural and spinal | |
| Refractory cardiac arrest with 0.75% concentration. Interaction with cardiac Na+ channels “fast in, slow out” | |
| Disruption of atrioventricular nodal conduction | |
| Depression of myocardial contractility | |
| Indirect effects mediated by CNS | |
| Limitations on total dose of bupivacaine given | |
| Ropivacaine | Developed due to cardiotoxicity related to bupivacaine |
| Long-acting | |
| Slightly less potent than bupivacaine | |
| Higher concentrations fastens its onset and density of block | |
| Reduced CNS/CV toxicity compared to bupivacaine |
The choice of local anesthetic affects the quality of the block, time to onset, and duration of action (Table 4.2).15 Quicker onset generally leads to quicker clearance. Lidocaine and mepivacaine, agents with intermediate duration, have a short latency period which is further shortened by the addition of bicarbonate as mentioned above. In comparison, bupivacaine and ropivacaine, two long acting agents used commonly in regional anesthesia, have a longer latency period, and are not able to mix with bicarbonate due to precipitation concerns. In order to obtain both the quicker onset characteristic and the longer duration, some may consider mixing two agents together to achieve the quicker onset effect and the longer duration of anesthesia/analgesia. These mixtures can be about 50 : 50, however, it can vary depending on the experience and training of the anesthesiologist. The toxicity of mixtures are additive and the mixture does not lower the overall toxicity.17
Another consideration is the differential blockade, as nerves are blocked unequally and at different rates. Nerve blockade proceeds in the following order: sympathetic nerves, pin-prick sensation, touch, temperature, and finally motor.18 This is an important attribute of bupivacaine, as one can provide improved analgesia without much motor blockade in the postoperative period if an infusion is run at analgesic doses. Optimally, an LA with sensory selectivity is desired.
Regional anesthesia techniques
Regional anesthesia has been shown to be an excellent anesthetic modality for upper extremity surgery. This relates to long lasting pain relief, reduced opioid-related side-effects during the first 24 hours after surgery, and expedited hospital discharge.2,19 Despite this, many patients still receive other types of anesthesia for a variety of reasons. Alternatives to regional anesthesia include general anesthesia, monitored anesthetic care (MAC), Bier block or simple local anesthetic infiltration without blockade of the brachial plexus. The factors involved in determining suitability of an anesthetic include patient preference, surgeon preference, relative and absolute contraindications to regional anesthesia, as well as type of surgery. General anesthesia has been utilized for many years with a safety record that has improved significantly over the past decades.20 Due to respiratory depression, general anesthesia requires airway management not routine in regional anesthesia, local anesthesia or in monitored anesthetic care. Additionally, patients who undergo general anesthesia may experience hemodynamic variations that may be significant in those with cardiac disease. All patients who will undergo any type of anesthesia need to have standard ASA monitors, which include pulse oximetry, blood pressure monitoring, and electrocardiogram monitoring as well as intravascular access established in the nonoperative limb.
The use of ultrasound guided blocks has gained significant momentum in the last decade. The benefits of ultrasound include shortened time to onset, enhanced visualization of the nerve target and surrounding structures such as arteries, veins, muscle, and other soft tissues, needle visualization, visualization of the local anesthetic and its spread, and anomalies of anatomy.21 Combining the traditional technique of peripheral nerve stimulation with ultrasound has not demonstrated notable benefits, although that has been a common practice, particularly for difficult cases. Surprisingly, there have been no studies that demonstrate improved safety with ultrasound over the technique of peripheral nerve stimulation.22,23
Digital block
The use of epinephrine in digital blocks has been a controversial subject. Despite the admonition against epinephrine use in numerous medical textbooks, no case of digital gangrene has been reported in the literature resulting solely from the use of epinephrine with a local anesthetic. A number of studies have demonstrated epinephrine can be safely used as an adjunct for digital block anesthesia. Lalonde et al. performed a randomized, prospective, blinded study with over 3000 consecutive cases and showed no cases of infarction, necrosis or tissue loss.24 Epinephrine can be added to local anesthetics to lengthen the duration of action, lessen bleeding, reduce the need for a tourniquet, and reduce the risk of adverse systemic effects.25