L’Oreal USA
111 Terminal Ave
Clark, NJ
Contributing Authors:
Peter Konish
Director of Sensory and Formulation Development
NeoStrata Company, Inc
307 College Road East
Princeton, NJ 08540
Stacy S. Hawkins, Ph.D.
Global Clinical Leader
Unilever Research and Development
50 Commerce Drive, Trumbull, CT 06615
Uma Santhanam, Ph.D.
Senior Manager, Cell Biology and In Vitro Toxicology
Avon Products, Inc.
One Avon Place
Suffern, NY 10901
aNeoStrata Company Inc, Princeton, NJ 08540 USA
bUnilever R&D, Trumbull, CT 06611 USA
cAvon Products Inc, Suffern, NY USA
ABSTRACT
The alpha-hydroxy acids (AHAs), including glycolic acid, are ingredients that have revolutionized cosmetic anti-aging and are a critical part of the cosmetic chemist’s formulary. This class of small organic acids has significant reparative effects to all layers of the skin, including exfoliation of the stratum corneum, increased epidermal keratinocyte proliferation and differentiation, modulation of melanin production, and increase to fibroblast production of dermal matrix components, resulting in clinically proven and consumer-recognized cosmetic benefits collectively associated with anti-aging.
Second- and third-generation hydroxy acids, including polyhydroxy acids (PHAs) and bionic acids (BAs), are not only milder having increased tolerability, but also impart protective effects such as barrier strengthening, antioxidant, and matrix metalloproteinase inhibition. More recently, acetylamino sugars and acetylamino acid derivatives have arisen from the AHA research. Many of these compounds are effective at higher pH, and like the PHAs, provide significant anti-aging benefits with inherent gentleness to the skin. Formulating with hydroxy acids represents a challenge to the cosmetic chemist, as there are nuances to formulating for efficacy without irritation, and in addition, low-pH emulsions can be difficult to stabilize. However, the challenges to formulating highly effective and stable topical products containing hydroxy acids and related compounds can be overcome through careful selection of vehicle components, including thickeners, emulsifiers, formula pH, and ingredient combinations.
This chapter provides an in-depth review of the evolution and discovery of the value of AHAs as uniquely powerful anti-aging ingredients. The in-depth review of the literature, combined with numerous clinical study results and the thoughtful description of the various mechanisms proposed, is compelling. Also of interest is the description of various cogent formulation approaches to maximizing efficacy while minimizing irritation.
4.3.5.2 Alpha-Hydroxy Acids (AHAs)
4.3.5.3 Quantitative Clinical Benefits of AHAs
4.3.5.4 Cellular and Structural Changes Associated with AHAs
4.3.5.5 Polyhydroxy Acids (PHAs)
4.3.5.7 Formulation Strategies for Maximizing Hydroxy Acid Performance
Improvement in the appearance of photo-aged skin continues to be a high priority among consumers. Photo-aged skin is the manifestation of accumulated skin damage from chronic sun exposure superimposed upon the chronological aging process. The appearance of photo-aged skin is typically defined by lines and wrinkles, irregular texture, loss of firmness and elasticity, and an increased amount and reduced uniformity of pigmentation. The alpha-hydroxy acids (AHAs) were initially discovered by Drs. Van Scott and Yu in the 1970s and found to be useful for their effects on normalizing hyperkeratotic conditions such as ichthyosis.1 In the subsequent decades, the discoveries of the broad pleiotropic effects of AHAs on all layers of the skin resulted in an array of cosmetic clinical benefits that is unmatched in cosmetic skin care. Topical cosmetic formulations containing AHAs at low concentrations (4–10%) have been shown to improve the appearance of photo-damage by a number of quantitative clinical endpoints. Numerous investigations into the mechanisms of action of AHAs support that the cosmetic benefits are likely derived from their impact on the skin surface as well as well as stimulatory effects on keratinocytes and fibroblasts.
The first-generation AHAs gave way to second- and third-generation polyhydroxy acids (PHAs) and bionic acids (BAs), which impart comparable clinical benefits for amelioration of photo-aging to AHAs, with improved mildness and additional skin-protective effects. This stream of investigation led to the discovery of the anti-aging benefits of N-acetylamino sugars and N-acetylamino acids and derivatives. The majority of these compounds are physiologic, natural, and nontoxic. They normalize keratinization and skin structure. The compounds discussed herein have a marked effect on epidermal and dermal thickness, and stimulate dermal fibroblasts to increase synthesis of glycosaminoglycans and collagen. Those with multiple hydroxyl groups impart a humectancy benefit as well as antioxidant characteristics, and are ideal for cosmetic treatment of aging skin appearance. This latest generation of anti-aging ingredients are especially gentle, which makes them ideal for use on sensitive skin.
Successfully formulating with AHAs requires a multiplicity of considerations since the balance of efficacy and irritation can be dependent upon the type of formula employed (such as oil-in-water, water-in-oil, or water-in-silicone emulsions) and the desired pH. Further, other parameters to be considered are emulsifier and thickener selection in order to ensure formula stability, and selection of co-actives, which work optimally at a given pH. Thus, the thoughtful formulator must balance a variety of critical parameters in maximizing performance of cosmetic formulations containing hydroxy acids.
4.3.5.2 ALPHA-HYDROXY ACIDS (AHAS)
Structural and Physicochemical Properties of AHAs
The AHAs are organic carboxylic acids, with the distinct feature of having a hydroxyl functionality group attached to the carbon unit adjacent (or in the alpha position) to a carboxylic acid moiety.
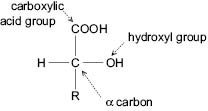
Figure 1: Alpha-Hydroxy Acid Chemistry
The AHAs are typically low-molecular-weight compounds with acidity that is modulated by the proximal hydroxyl group. AHAs are found naturally in many fruits, and have aptly been nicknamed the fruit acids.
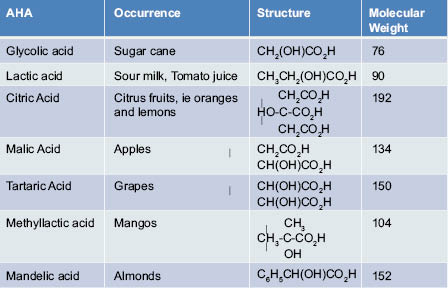
The most commonly used AHAs in skin care today include glycolic acid, lactic acid, and citric acid. Glycolic acid, or 2-hydroxyethanoic acid, is the smallest of the AHA family, and is found in sugar cane juice. Glycolic acid has a molecular mass of approximately 76 daltons and a pKa of 3.8, approximately 1 unit lower than its nonhydroxy analog acetic acid. Lactic acid, or 2-hydroxypropanoic acid, is a 3-carbon AHA derived from milk. Lactic acid is endogenous to the body and is produced via metabolism and exercise, during which time pyruvate becomes converted by the enzyme lactate dehydrogenase. Several AHAs, such as lactic acid, have chiral carbon atoms, resulting in D and L stereoisomers. Citric acid is a polycarboxyl AHA, and is a unique hydroxy acid in that it has a hydroxyl group alpha to the 2-carboxylic acid moiety, and beta to the 1- and 3-carboxylic acid moieties. This structural configuration thereby makes it not only an AHA but also a beta-hydroxy acid (BHA).
Citric acid is naturally found in citrus fruits, and is also found in the body in the Kreb’s (citric acid) cycle where it is critically involved in energy production. Citric acid also has antioxidant properties. These smaller AHAs are hygroscopic in nature and highly soluble in water. Mandelic acid, or phenylglycolic acid, is an aromatic alpha-hydroxy acid, and, as such, is considered a lipophilic AHA. As a lipophilic AHA, mandelic acid tends to not only possess anti-aging benefits, but has unique applications for oily and acne-prone skin due to the affinity of more lipophilic molecules to sebum.
Table 1: Alpha-Hydroxy Acids – Natural Occurrence and Properties
Discovery of AHA Benefits
The cosmetic uses of AHAs are said to date back to the times of Cleopatra, renowned queen of ancient Egypt, where the benefits of milk acids, later to be identified as lactic acid, were known at this early time for generating younger smoother skin. However, key scientific and clinical evidence for AHAs emerged in the 1970s when Van Scott and Yu discovered the effects of glycolic acid on normalizing skin keratinization dysfunctions in hyperkeratotic conditions such as ichthyosis.1
Through decades of clinical, histological, and cellular investigations, the beneficial effects of glycolic acid on stratum corneum desquamation and plasticization, epidermal proliferation and signaling, dermal matrix remodeling, and melanin production were discovered, providing strong support for the potential use of this compound for improving photo-aged skin. Initially the benefits of 25% glycolic acid for reversing both epidermal and dermal markers of photo-aging were demonstrated on photo-damaged forearms,2 and subsequently the efficacy of lower-strength glycolic acid formulations were demonstrated.3 As a result, glycolic acid has become one of the most widespread cosmetic anti-aging ingredients used.
4.3.5.3 QUANTITATIVE CLINICAL BENEFITS OF AHAS
The clinical benefits of topical cosmetic AHAs have been demonstrated in numerous randomized, double-blind, vehicle-controlled studies in both face3,4 and photo-damaged arm5,6 models. A few of the many clinical studies are described below.
The first such study of cosmetic strength AHAs was a 22-week, single-center study with both 8% glycolic and 8% L-lactic acid compared to a vehicle control.3 Products were applied monadically to the entire face and in paired comparison application to photo-damaged arms. Significant benefits to overall severity of photo-damage were observed with both glycolic and lactic acid compared to the vehicle control on the face and arm. Further, evaluation of the photo-damaged forearm provided greater sensitivity for differentiating individual aspects of photo-damage, such as the appearance of mottled hyperpigmentation, sallowness, and fine lines and wrinkles.
Another randomized, double-blind, vehicle-controlled face and neck 12-week application study compared a 5% unneutralized formulation of glycolic acid to its vehicle control.4 In this study, the glycolic acid treatment showed significant improvement compared to the vehicle control in physician-assessed skin texture, and a strong trend for improvement to discoloration (composite score of melasma, solar lentigines, guttate hypomelanosis, poikiloderma) compared to the vehicle control.
Additional studies have shown benefits of AHAs in both paired forearm application and split-face application studies.7-10 In addition to expert visual assessment of photo-damage, objective instrumental assessments have shown additional clinical benefits of AHAs compared to vehicle controls, and allow for sensitive measurement of their benefit at earlier time points. In 12-week photo-damaged arm studies,5-7 healthy female subjects (n~20 per product application cell), ages 45–70 years old, with a moderate degree (grades 4–6 on a 0–9 scale) of photo-damaged skin on the forearms, were enrolled to participate in IRB-approved arm studies. Photo-damage on the forearms was defined by the appearance of “crepe-like” skin,5 using a 0–9 scale (0 = none, 1–3 = mild, 4–6 = moderate, 7–9 = severe, with ½ point grades allowed). In addition, the arms were compared to obtain a directed difference assessment, using a 0–4 scale, of skin tone, dryness, crepey appearance, and overall photo-damage. The forearm studies were randomized 12-week, double-blinded, bilateral-paired comparison studies. Subjects applied 0.5 to 1.0 g of each product to the appropriate left/right forearm, twice daily for 12 weeks. Efficacy was assessed at baseline and after 4, 8, and 12 weeks of product application by an expert evaluator using the grading scales shown in Tables 2–4 below.
Table 2: Photodamage Intensity of Crepe-Like Appearance5
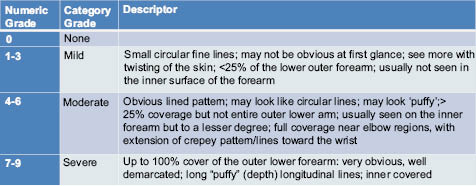
Table 3: Directed Difference Attributes for Photo-Damaged Arm Appearance5

Table 4: Directed Difference Magnitude for Photo-Damaged Arm Appearance5

The benefits of 8% glycolic acid cream (pH 3.8) compared to a vehicle cream were assessed in repeated clinical studies using the Photo-Damaged Arm Model.5 Improvement by glycolic acid to the crepe-like appearance of skin was statistically significant in 15 out of 16 clinical studies. The reproducibility of the magnitude of benefit imparted by glycolic acid was demonstrated within individual studies and across studies. In a similar study design, the significant improvement of an 8% glycolic acid (pH 3.8) compared to its vehicle cream was observed for crepe-like skin intensity (see Figure 2). Glycolic acid provided significantly greater improvement to crepe-like skin appearance than its vehicle after 8 and 12 weeks of use. This was also demonstrated using the directed difference evaluation of overall appearance and crepe-like appearance after 8 and 12 weeks of application, and continued improvement at 12 weeks of application. A trend was observed (p<0.1) for directed difference evaluation of skin tone (Figure 3).
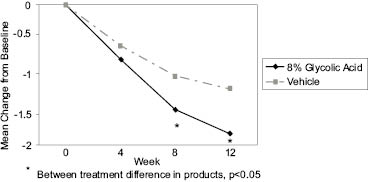
Figure 2: Improvement (from Baseline) to the Intensity of Crepe-Like Appearance on Forearms: 8% Glycolic Acid Cream Compared to Vehicle
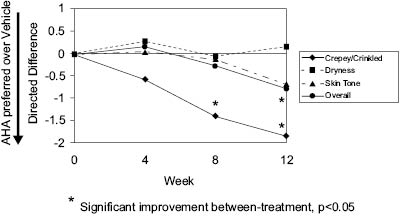
Figure 3: Directed Difference Evaluation of Photo-Damaged Arms: 8% Glycolic Acid Cream Compared to Vehicle
In split-face photo-damaged skin studies,7-10 healthy female subjects, ages 30–70 years old, with moderate to severe overall facial photo-damage, were enrolled to participate in IRB-approved vehicle-controlled facial studies. Since subjects served as their own within-treatment control (e.g., applied both the AHA and vehicle cream to either side of their face), these studies required fewer subjects than monadic application studies, where independent groups of subjects evaluated either the test product or the control on the whole face. In addition, the introduction of noninvasive, objective instrumental evaluation to the clinical protocols allowed for more sensitive discrimination between the effects of the test products versus the vehicle, as well as detection of effects at earlier time points. Therefore these studies were typically a maximum of 12 weeks in duration, shorter than the earlier monadic studies, which were six months in duration.3
Photo-damage intensity on the face was assessed using validated expert visual scales. Subjects applied test products to their face, twice daily for up to 12 weeks. Assessment of skin condition (overall photo-damage, lines and wrinkles) was conducted by expert visual assessment and objective instrumental measurements were obtained at baseline and at monthly intervals.
In the photo-damaged facial studies, most study expert visual assessments were made using traditional photo-numeric 0–9 scales, where 0 = none, 1–3 = mild, 4–6 = moderate, and 7–9 = severe.11–14 These scales are helpful for characterizing overall photo-damage as well as individual attributes of photo-damage, and assessments may be made using ½ point increments,14 which allows for sensitive differentiation between technologies. In one study, 10 visual parameters were assessed using a physician global assessment scale, where 0=none, 1=mild, 2=moderate, and 3=severe. All ten parameters were summed to provide an overall score, as well as a wrinkle and discoloration score (the sum of four out of ten parameters for each).4
In addition to visual assessment of photo-damage, objective instrumental assessment of skin improvement by AHAs included digital photography, ballistometry (elasticity and firmness), and 3D Cyberware™ facial scanning. The Diastron™ ballistometer is a pendulum-based, torsional instrument that can be applied to the skin at different forces, to measure initial indentation and rebound from the skin, as well as a measure of overall elasticity (damping coefficient, alpha). The 3D 3030/HIREZ Cyberware™ facial scanner uses a near-IR line projector attached to a cylindrical motion stage to compute 3-D depth information on the face in cylindrical coordinates (Figure 4). From these virtual scans of the face, the length, depth, and volumes of surfaces may be calculated. Since the development of this technique, there are now other commercially available 3-D face and body systems. Over time, these systems have continued to improve the resolution and photorealism of 3-D images.
Figure 4: Sample C3030/HIREZ Cyberware™ Facial Scanner Images
In a previously reported split-face and photo-damaged arm application study,7 subjects applied an 8% glycolic acid cream (pH 3.8) compared to its vehicle. Ballistometer readings were collected at weeks 0, 4, and 8 using three different force settings. The ballistometer response curves were analyzed with digital filtering (Fast Fourier Transform methods) to remove noise, and then a genetic algorithm (GA) was used to identify features in the filtered response curves. Both the glycolic acid cream and vehicle cream improved skin elasticity at weeks 4 and 8. The GA was able to correctly classify an “active” response on the glycolic-treated side over a vehicle response in 17 out of 18 subjects, in both the face and arm treatment sites.
The 3-D depth and length of fine lines and wrinkles around the periorbital region were evaluated using the Cyberware™ 3030 HIREZ facial scanner in a split-face application study (P. D. Hawkins SS). An 8% glycolic acid cream (pH 3.8) was compared to its vehicle cream. Significant improvement to the length of fine lines was observed after four weeks of product application with the glycolic acid cream (Figure 5). The glycolic acid cream was statistically superior to the vehicle cream at weeks 4 and 8 for the length of fine lines and wrinkles, and at week 8 for the depth of fine lines and wrinkles. A sample baseline and week 8 image from the 2-D photographs is shown in Figure 6.
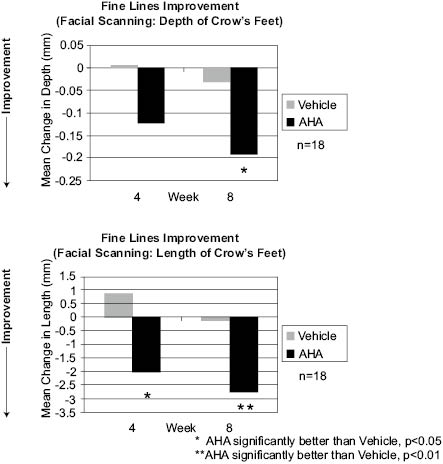
Figure 5: 3D evaluation of lines and wrinkles in the periorbital site using the Cyberware™ 3030 HIREZ facial scanner.
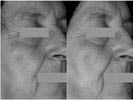
Figure 6: Left) Baseline; Right) After eight weeks of application using 8% glycolic acid cream (pH 3.8). After treatment, the fine lines and wrinkles are fewer and less deep in the periorbital area. The skin tone is also improved on the cheek, and the overall texture is smoother.
Consumers are also able to perceive the skin benefits imparted by AHAs over the vehicle controls. In a previously published report,9 subjects viewed before and after photos in a randomized, double-blind 12-week multicell split-face application study. One of the treatment groups compared an 8% glycolic acid cream to its vehicle. Consumers evaluated their skin condition across multiple anti-aging attributes at the end of the study without using photos. Approximately three months later, subjects returned to view their baseline and week 12 photos, and were administered a similar questionnaire using the same attributes previously evaluated. Subjects were able perceive improvement to photo-damaged appearance, such as lines and wrinkles, evenness of color, and firm appearance, which were statistically significant in favor of the AHA cream compared to its vehicle.
The anti-aging benefits of AHA formulations over a vehicle control have been observed in multiple randomized, double-blind, vehicle-controlled studies. In both the split-face photo-damaged face and paired comparison arm models, improvement to appearance attributes has been observed for texture/lines and wrinkles as well as even tone/hyperpigmentation and overall appearance. These clinical changes correspond to the longer-term benefits observed in earlier six-month monadic use clinical studies, and translate to consumer perceived benefits, as observed in split-face application photo-damaged facial tests.
4.3.5.4 CELLULAR AND STRUCTURAL CHANGES ASSOCIATED WITH ALPHA-HYDROXY ACIDS
Several studies have been conducted to understand the modes of action by which AHAs provide their beneficial effects on skin. These studies have demonstrated that AHAs can have effects on all layers of the skin, starting with the outermost layer, the stratum corneum. The effects of AHAs extend through the viable epidermis, to the dermal compartment. In vitro studies have indicated that AHAs can have direct effects on multiple cell types present in the skin, including keratinocytes, fibroblasts, and melanocytes, which may explain, in part, the clinically observable effects on skin such as moisturization and improvement of photo-aging.
Effects of AHAs on the Stratum Corneum
Early studies by Van Scott and Yu1,15 demonstrated that treatment of xerotic skin results in the normalization of stratum corneum (SC) exfoliation, increased plasticization, decreased formation of flaky scales, and a thinner, flexible, and more compact stratum corneum. An extreme example of this effect is seen in Figure 7, where thick scaly plaque skin treated with a combination of 20% alpha- and polyhydroxy acids (pH 3.8) provides a dramatic improvement to this condition.
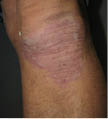
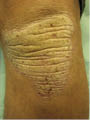
Figure 7: Descaling effect observed with 20% AHA/PHA/bionic acid formulation: scaling on knee before (left) and after use (right)
One of the ways in which AHAs are believed to bring about these effects is by diminishing the adhesiveness of the corneal layer. AHAs are believed to modulate desquamation by altering or degrading corneosomes (desmosomes), thereby decreasing cohesion between corneocyte layers. There is general agreement that this action is restricted to the stratum corneum, but where exactly the AHAs exert their exfoliating effect in this epidermal layer remains unclear.
In low concentrations, for example 2–5%, glycolic acid is believed to facilitate progressive weakening of cohesion of the intercellular material of the stratum corneum, resulting in uniform exfoliation of its outermost layers, the stratum disjunctum. A study by Fartasch et al.16 investigated the effects of topical treatment with a 4% glycolic acid formulation, pH 3.8, on the SC using electron microscopy. The lamellar body (LB) secretory system in the stratum granulosum (SG), and intercellular lipid lamellae in the SC in both vehicle and glycolic acid-treated samples were found to be comparable to normal human SC. The site of action of glycolic acid as applied in this study appeared to be the desmosomes.
Normally, progressive stages of degradation of the desmosomal plug are seen as a function of their precise location in the different SC layers.17 The skin’s natural degradation in the stratum disjunctum was accelerated in the glycolic acid-treated skin compared with vehicle-treated skin. Within the SC, enhanced desmosomal breakdown, promoting loss of cohesion and desquamation, appeared to be restricted to the stratum disjunctum while desmosomes of the stratum compactum were unaffected. In this study, glycolic acid appears to target the desmosomes without affecting the barrier structures of the stratum corneum since TEWL (trans-epidermal water loss) values remained unchanged. It also appears that lactic acid can stimulate ceramide synthesis,18 which suggests that AHAs may provide improvement in skin barrier function.
Several hypotheses have been proposed with regard to the mechanisms by which AHAs affect the cohesion of the layers within the stratum corneum and enhance desquamation. AHAs could affect ionic bonding of the layers, inhibit enzymes such as sulfate and phosphotransferases, resulting in fewer electronegative sulfate and phosphate groups on the surface of corneocytes, thus diminishing cohesive forces.15 Enhanced desquamation may also be achieved via preferential acidification of the polar domains embedded within the hydrophilic lipid bilayers, or by activating by other means the acid proteases crucial for desmosomal degradation.19 Human tissue kallikreins are a family of 15 trypsin- or chymotrypsin-like secreted serine proteases (KLK1–KLK15). Many KLKs have been identified in normal stratum corneum (SC) and are candidate desquamation-related proteases, particularly KLK-7.20 AHAs could transiently change the pH of the corneum layers and activate these proteases, leading to corneosome dissolution. Topical application of a high concentration (50% @ pH 0.9) led to an initial inactivation followed by prolonged activation of cathepsin D-like protease in the upper layers. No changes in chymotrypsin-like proteinases were observed in this study.21
When applied to hairless mice, high concentrations of AHAs (70% glycolic acid) resulted in a loss of calcium gradient. This could lead to lamellar body exocytosis. An in vitro study using cultured keratinocytes suggested that glycolic acid could lower the calcium ion concentration, at least in part, through the chelating effects of the glycolic acid on the cationic ions.22
Effect of AHAs on the Epidermis
One of the most consistent effects observed following topical application of AHAs is an increase in viable epidermal thickness. Lavker et al.23 reported that daily topical application of a 12% ammonium lactate formulation resulted in an increase in epidermal thickness. A thickened epidermis was also observed following 5% or 12% lactic acid application twice daily for three months.24 Similar results were reported with a three-week application of glycolic acid.25
This change in the epidermis of skin could be a direct effect of AHAs on keratinocyte proliferation. When human epidermal keratinocytes were exposed to glycolic acid and L-lactic acid in cell culture, a stimulatory effect on keratinocyte proliferation was observed. The results of these studies indicated that both glycolic and lactic acids are mitogenic in nature as each significantly stimulated proliferation of keratinocytes compared to control cells.26
Effect of AHAs on the Dermis
In addition to the effects on the stratum corneum and the viable epidermis by AHAs, beneficial changes in the dermis have also been reported in multiple studies. These effects include a marked increase in glycosaminoglycans (GAGs), collagen, and dermal thickness. The studies described below used a range of concentrations of AHAs, starting from 4% to up to 50% and at varying pHs. Lavker et al.23 demonstrated that 12% ammonium lactate, applied to normal skin in an open (four weeks) or occluded (three weeks) manner, resulted in a striking deposition of GAGs in the dermis. This AHA preparation also spared the atrophic effects of corticosteroids on the epidermis and dermis when applied in combination with clobetasol propionate.
In addition to GAGs, increased deposition of collagen has also been observed following application of AHAs. Smith et al. reported that treatment with 12% lactic acid resulted in increased epidermal and dermal firmness and thickness. This was accompanied by clinical improvement in skin smoothness and in the appearance of lines and wrinkles. In the same study, a lower concentration of AHA, namely 5% lactic acid, modulated surface and epidermal changes but no dermal change.24
In another study, 4% glycolic acid, pH 3.8, in a suitable formulation, was applied to forearms of ten subjects for three weeks under semi-occlusive conditions (five patches/week for three weeks). Biopsies were collected at the end of three weeks and samples were analyzed for viable epidermal thickness, pro-collagen 1 by immunohistochemistry, total collagen (Masson trichrome stain) and GAGs (colloidal iron). Four out of ten subjects showed an increase in viable epidermal thickness and in colloidal iron staining and seven out of ten showed increases in pro-collagen 1 as well as total collagen.27 Representative images are shown in Figures 8–11.
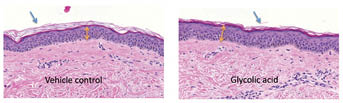
Figure 8: Effect of topical application of glycolic acid on viable epidermis. The dorsal forearms of ten subjects were each treated with 4% glycolic acid formulation or vehicle control for three weeks under semi-occlusive patch. Treated sites were biopsied and sections were stained with hematoxylin & eosin. Viable epidermal thickness (VET) was increased in four out of ten subjects (yellow arrows). Stratum corneum layers were reduced in eight out of ten subjects (blue arrows). Epidermal cells became more “compact” and “organized” in subjects with increased VET.
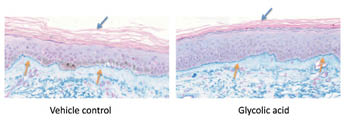
Figure 9: Effect of topical application of glycolic acid on glycosaminoglycans (GAGs). The dorsal forearms of ten subjects were each treated with 4% glycolic acid formulation or vehicle control for three weeks under semi-occlusive patch. Treated sites were biopsied and sections were stained with colloidal iron stain. Four out of ten subjects showed increased colloidal iron deposition at DEJ, the staining pattern became thicker and more continuous (yellow arrows).
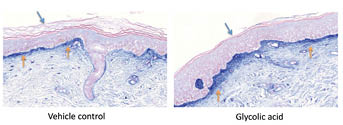
Figure 10: Effect of topical application of glycolic acid on pro-collagen 1. The dorsal forearms of ten subjects were each treated with 4% glycolic acid formulation or vehicle control for three weeks under semi-occlusive patch. Treated sites were biopsied and sections were stained for immunohistochemical analysis for pro-collagen-1, a biomarker for new collagen synthesis. Five out of ten subjects showed increased pro-collagen-1 staining level at papillary dermis (yellow arrows). There was a significant induction of pro-collagen-1 at papillary dermis and dermal-epidermal junction, indicating a beneficial biochemical effect of topical AHA treatment.
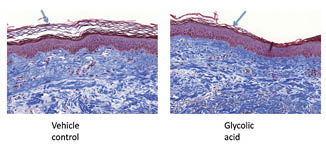
Figure 11: Effect of topical application of glycolic acid on total collagen. The dorsal forearms of ten subjects were each treated with 4% glycolic acid formulation or vehicle control for three weeks under semi-occlusive patch. Treated sites were biopsied and sections were stained with Masson trichrome stain. Seven out of ten subjects showed increased intensity of fiber-forming collagen staining, indicating a beneficial biochemical effect of topical AHA treatment.
In the pivotal 8% AHA clinical study by Stiller et al.,3 where the clinical improvement to photo-damaged skin after 22 weeks was first reported, histological changes in skin were also observed.28 In this study, biopsies at baseline and week 22 were obtained and immunohistochemistry for pro-collagen 1 was performed. Slides were blindly evaluated for changes in pro-collagen staining, and an increase in pro-collagen 1 staining intensity relative to baseline was observed in all three groups—vehicle, glycolic, and lactic acid-treated skin samples. While no significant difference was observed between the groups, there was a significant correlation between collagen synthesis and changes in clinical photo-aging, suggesting that clinical improvement after AHA/sunscreen treatment may be due, in part, to increased collagen synthesis. Examination of the samples using electron microscopy revealed that dermal density and papillary dermal collagen structures increased in all three groups, correlating with immunohistochemistry results. Increase in anchoring fibrils and dermal density correlated with clinical improvement. This was the first time electron microscopy results demonstrated treatment-induced structural changes in photo-damaged skin, that were consistent with clinical improvement.
Bernstein et al.29 performed a study employing a higher concentration of AHA, 20% glycolic acid, twice a day for three months, and demonstrated an increase in collagen gene expression and additionally, increases in epidermal and dermal hyaluronic acid production. In addition to glycolic and lactic acids, other AHAs, such as citric acid, having clinical benefits for photo-aging skin, have also been studied for their effect on dermal matrix components. Bernstein et al.30 observed that 20% citric acid application for three months resulted in increased viable epidermal thickness and dermal glycosaminoglycans in AHA-treated skin, compared to vehicle-treated site.
A study by Ditre et al.2 used even higher concentrations of AHAs, e.g., 25% glycolic, lactic or citric acid and demonstrated that topical application of these AHA lotions for an average of six months caused a 25% increase in skin thickness relative to placebo. This increase could be attributed to the increased thickness of the epidermis as well as the dermis, and papillary dermal changes included increased acid mucopolysaccharides, improved quality of elastic fibers, and increased density of collagen. Induction of epidermal cell proliferation, correction of epidermal basal cell atypia, enhanced melanin pigment dispersal, and a return to a more undulating rete pattern at the epidermal-dermal junction were also reported.
Mechanism of Action Studies In Vitro
In order to further understand the dermal remodeling action of AHAs observed in vivo, several studies looked at the effects of AHAs on fibroblasts in vitro. Human dermal fibroblasts exposed to glycolic and L-lactic acids in cell culture secreted significantly higher levels of pro-collagen 1, and this response was dose dependent.26 Two other studies31,32 also reported similar effects, using 3H-proline labeling, suggesting that AHAs have a stimulatory effect on collagen at the protein synthesis level, and glycolic was found to be superior to malic acid in stimulating collagen. It has been suggested that the net increase in collagen observed in vitro may be, in part, due to a primary effect on fibroblast proliferation.33
Okano et al.34 investigated the effect of glycolic acid on the dermal matrix metabolism of keratinocytes and fibroblasts using in vitro and ex vivo systems. Glycolic acid was found to directly accelerate collagen synthesis by fibroblasts in a dose-dependent manner. Keratinocytes treated with glycolic acid released higher levels of IL-1 alpha, and this effect was also observed ex vivo.35 When fibroblasts were exposed to conditioned medium from keratinocytes treated with glycolic acid, they up-regulated MMP-1 and MMP3 mRNA expression. Direct treatment of fibroblasts to glycolic acid did not elicit this response. The effect on MMP expression is likely mediated by IL-1alpha, which was up-regulated by glycolic acid in keratinocytes. Rakic et al.36
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








