Fig. 9.1
Pathology of skin acute rejection. Illustration of the VCA Banff 2007 classification. a Grade 1, mild rejection. Pathological appearance of the sentinel skin graft of the first face transplant patient 6 years after transplantation (patient 7) shows mild perivascular infiltration without involvement of the overlying epidermis. b Grade 2, moderate rejection. Skin biopsy of the forearm in a bilateral hand transplant patient 2 years after transplantation (patient 5). Pathological appearance of the skin shows moderate-to-severe perivascular inflammation without epidermal involvement. c Grade 3, severe rejection. Skin biopsy on facial allograft 2.5 years after transplantation (patient 8). Pathological appearance of the skin shows lichenoid epidermal hyperplasia and lymphocyte infiltration in the upper dermis. The epidermis contains lymphocytic exocytosis. VCA vascularized composite allotransplantation
The role of B cells in acute skin rejection is uncertain. B cells are poorly represented within skin infiltrates (Fig. 9.2, [8, 10]). Circulating donor-specific anti-human leukocyte antigen (HLA) antibodies (DSA) have not been durably detected in hand transplant recipients, despite a high incidence of AR episodes [10, 14, 15]. The patient from the Louisville group who lost his grafted hand from vascular rejection had no DSA at the time of graft loss. Interestingly, DSA appeared 2 days after amputation [6], suggesting that anti-HLA antibodies could have been sequestered in the graft, as observed in kidney transplant recipients who developed DSA after transplant nephrectomy [16, 17]. The presence of C4d deposits in the skin and their significance as a marker of antibody-mediated rejection (AMR) remains doubtful. In kidney transplantation, deposition of the C4d complement degradation product in the capillary lumen is a relevant marker of AMR [18]. In our experience of hand and face transplantation, such deposits are exceptionally, if ever, detected in the allografted skin and mucosa [19]. Additionally, intraluminal capillary C4d deposits have been reported by other groups in the skin component both with and without concomitant signs of AR, DSA, and B cell infiltration [20–22]; this questions the usefulness of C4d detection in diagnosing VCA rejection. Besides producing antibodies, B cells might be involved in skin rejection through their ability to secrete cytokines and to act as antigen-presenting cells. B cells are indeed key components to generate memory CD4 + T cells [23].
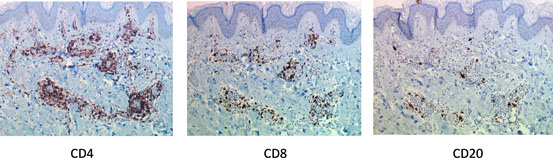

Fig. 9.2
Immunohistochemical analysis of skin rejection in hand transplantation. Immunohistochemical studies showed that dermal-infiltrating lymphocytes expressed predominantly a CD3 + /CD4 + phenotype, with fewer cells expressing the CD3 + /CD8 + phenotype. CD20 + B cells are scarcely represented. (Hand transplant biopsy at 2 years posttransplantation, patient 5)
Altogether, these data suggest that the humoral arm of immune response does not play a significant role in skin AR in VCA.
Lymphocyte Migration to the Skin
Recruitment of immune cells to the skin graft involving chemokines and adhesion molecules is a key event in the process of AR as in other inflammatory cutaneous reactions [24, 25]. Expression of markers involved in cell trafficking has been extensively analyzed in protocol and for-cause hand transplant skin biopsies [10]. None of the markers investigated was found constitutively upregulated in the skin in the absence of histological signs of rejection. In contrast, the expression levels of lymphocyte function-associated antigen 1 (LFA-1), intercellular adhesion molecule 1 (ICAM-1), E-selectin, and P-selectin were upregulated upon rejection and correlated with the severity of rejection. This study also suggested that the superficial layers of the skin might be the primary sites of lymphocyte infiltration during the rejection process before their migration into the epidermis [10].
What Triggers Acute Skin Rejection in VCA?
Cellular rejection occurs as a result of an imbalance between the immunologic processes that maintain graft tolerance and those that promote graft rejection. One potential mechanism that could trigger such processes is activation of the innate immune response by ischemia/reperfusion injury (IRI) that subsequently initiates the adaptive alloimmune responses in the recipient. Emerging evidence has shown that innate immune activation as a consequence of IRI may occur in VCA [26, 27]. In addition, nonspecific stimuli such as mechanical trauma to the skin may also activate innate immune reactions [28]. Also viral infections, particularly cytomegalovirus (CMV) and herpesviruses, may trigger rejection episodes [9, 29].
Diagnosis of Skin Rejection
Frequency of Skin Rejection in the Clinical Setting
Although the immunosuppressive drugs currently used in solid organ transplantation usually ensure VCA viability , the majority of patients experience at least one episode of skin AR in the first posttransplant year. According to the International Hand and Composite Tissue Transplantation Registry [30], 85 % of hand-grafted patients and 54.5 % of face-grafted patients presented at least one episode of AR in the first posttransplant year, while multiple rejections developed in 56 % of them. Furthermore, steroid-resistant rejections are frequently observed, and require treatment with anti-thymocyte globulin (Thymoglobulin, Genzyme), basiliximab, or alemtuzumab (Campath-1H) [30]. Repeated episodes of skin rejection were observed in some patients beyond the first year after transplantation [30]. Table 9.1 shows the incidence of AR episodes in face and hand transplant patients in Lyon. All patients had at least one rejection episode in the first year, yet few patients experienced late AR episodes that required T-cell-depleting treatment by Thymoglobulin or Campath-1H.
Table 9.1
Incidence and severity of skin AR episodes in face and hand transplant patients. Lyon and Amiens experience
Patients | Sex | VCA | TR date (d/m/y) | No. of episodes | POD | Banff score | Initial IS treatment |
|---|---|---|---|---|---|---|---|
Patient 1 | M | Bilateral hands | 13.01.2000 | 2 | 53, 72 | 2, 2 | ATG, Tac, St, MMF |
Patient 2 | M | Bilateral hands | 30.04.2003 | 3 | 57, 86, 2759 | 2, 2, 2 | ATG, Tac, St, MMF |
Patient 3 | F | Bilateral hands | 19.02.2007 | 7 | 16, 271, 635, 951, 1365, 1855, 2250 | 2, 2, 3, 2, 3, 3, 3 | ATG, Tac, St, MMF |
Patient 4 | M | Bilateral hands | 4.07.2008 | 1 | 65 | 2 | ATG, Tac, St, MMF |
Patient 5 | M | Bilateral hands | 11.07.2009 | 3 | 10, 350, 560 | 2, 2, 2 | ATG, Tac, St, MMF |
Patient 6 | M | Bilateral hands | 05.11.2012 | 3 | 20, 88, 154 | 2, 3, 3 | ATG, Tac, St, MMF |
Patient 7 | F | Face | 27.11.2005 | 2 | 23, 214 | 2, 3 | ATG, Tac, St, MMF |
Patient 8 | M | Face | 27.11.2009 | 8 | 41, 112, 186, 239, 474, 527, 540, 931 | 3, 3, 2, 2, 3, 3, 3, 3 | ATG, Tac, St, MMF |
Patient 9 | F | Face | 13.06.2012 | 1 | 12 | 3 | ATG, Tac, St, MMF |
The high rate of AR episodes reported in this field of transplantation might be due to the prompt diagnosis of AR, as the corresponding lesions are easily seen, and to the high immunogenicity of the skin, as discussed above. Despite the high incidence of AR episodes, the graft survival rate was 96 % at 1 year, and no convincing evidence of chronic (skin) rejection was found in compliant recipients on long-term follow-up [15].
Positive Diagnosis of Skin Rejection
Acute skin rejection is diagnosed by visual inspection as it manifests clinically with erythematous macules, diffuse skin redness (Figs. 9.3 and 9.4), or asymptomatic papules over the allografted skin with or without burning pain. Lesions are usually distributed on the dorsum of hands and forearms and may be bilateral when numerous [8, 31–33]. All changes can be associated with limb edema. Atypical rejection that affects the skin of the palm and nail beds has also been reported. Nail lesions include leukonychia and dystrophy, sometimes resulting in nail loss [28].
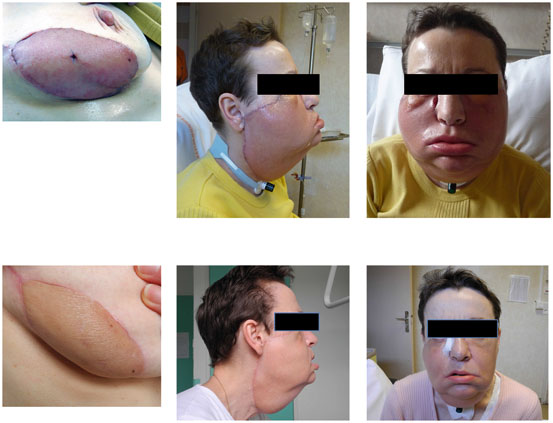
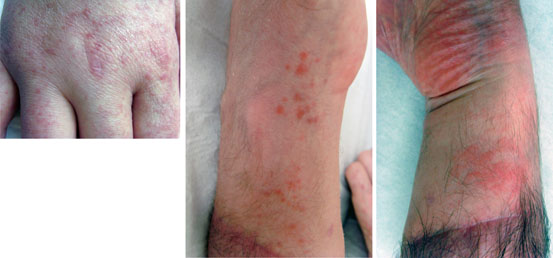

Fig. 9.3
Clinical aspects of skin rejection in face transplantation. Acute rejection grade 3, 12 days after face transplantation (patient 9): diffuse erythema on the sentinel skin graft (upper left panel); diffuse erythema and diffuse edema on the facial graft (upper right panel). The acute rejection episode was treated successfully with three boluses of intravenous methylprednisolone. The allograft skin of the sentinel skin graft (lower left panel) and the facial graft (lower right panel) shows normal appearance at 3 months posttransplantation

Fig. 9.4
Various clinical aspects of skin rejection in hand transplantation. Erythematous scaly papular lesions over the hand allograft, 5 years after bilateral hand transplantation (patient 3, left panel). Erythematous macula on the hand allograft during month 2 post-graft (patient 6, middle panel). Diffuse erythematous macula on the hand allograft during month 5 post-graft (patient 6, right panel)
Interestingly, skin rejection in face transplantation may have a different clinical presentation form than that of hand transplantation . In our experience, face rejection usually appears as diffuse redness combined with diffuse edema (Fig. 9.3 and [9]). The different aspect might be related to venous and lymphatic vascularization that differs between the hands and face, as during the same episode of rejection, the patients may present macular lesion of AR on the sentinel skin flap and diffuse redness and edema on the facial graft skin (Fig. 9.3).
Given the importance of visual inspection for the diagnosis of skin rejection , patients need to be educated for routine daily inspection of the graft, at least during the first year posttransplant. Since the clinical appearance is not specific, diagnosis of skin rejection has to be substantiated by histological examination, even though the pathological findings alone are not totally specific.
Pathology of Skin Rejection
Skin Biopsy
In contrast to renal and liver transplantation, where rejection can be suspected by serological biomarkers of organ dysfunction, histological examination of skin biopsies remains the only established technique for assessment of skin rejection in VCA . Skin biopsy is a nonrisky procedure and easy to perform using a 4-mm punch scalpel. An adequate sample should contain the epidermis, dermis, and some quantity of subcutaneous tissue (hypodermis) [34]. The main drawback of skin biopsy is the resulting scar that can affect the aesthetic outcome in particular in face transplantation. Biopsy of the cheek oral mucosa or the sentinel skin flap of donor origin has been used as an alternative in order to limit damage to the grafted face by repeated skin biopsies [35, 36]. The macroscopic features of the sentinel flap correspond well to those of the facial graft, and the pathological patterns of rejection are similar when skin biopsy specimens from both sites are compared [37]. Interestingly, the pathological patterns of rejection may appear earlier on the transplanted mucosa and also be more pronounced in the oral mucosa as compared with both the facial and the sentinel flap skin [37].
Pathology of Acute Skin Rejection
Microscopically, skin AR shows characteristic, although nonspecific, changes involving mainly the dermis and the epidermis that may also extend to the hypodermis in the case of severe rejection (Fig. 9.1). The earliest changes consist of a perivascular lymphocytic infiltrate in the superficial and mid dermis, predominantly made of CD3 + CD4 + and TIA-1 + cytotoxic CD8 + T cells, FoxP3 + T regulatory cells, and CD68 + histiomonocytic cells [8, 10]. In more severe rejection grades, this infiltrate may fill the dermis and invade the epidermis (exocytosis). The epidermis is initially spared in the early phase of AR. At later stages, it shows exocytosis and keratinocyte necrosis or apoptosis associated with basal keratinocyte vacuolization. More rarely the epidermis shows spongiosis (intercellular edema) or lichenoid changes (orthokeratotic hyperkeratosis, hypergranulosis, acanthosis, band-like subepidermal infiltrate), similar to those observed in (lichenoid) graft-versus-host disease (GVHD). In the case of very severe rejection, the epidermis (and its appendages, hair follicles, and sweat glands) may show extensive necrosis. In those severe cases, the infiltrate may extend to the hypodermis and contain also eosinophils. On the basis of these changes, a specific score (Banff score 2007) has been established in order to assess the severity of AR (Table 9.2 and [33]).
Table 9.2
The BANFF 2007 working classification of skin-containing composite tissue allograft pathology. This system comprises the following five severity grades in order to assess the severity of acute rejection [33]
Grade 0 (no rejection) | No or rare inflammatory infiltrates |
Grade 1 (mild rejection) | Mild perivascular infiltration. No involvement of the overlying epidermis |
Grade 2 (moderate rejection) | Moderate-to-severe perivascular inflammation with or without mild epidermal and/or adnexal involvement (limited to spongiosis and exocytosis). No epidermal dyskeratosis or apoptosis |
Grade 3 (severe rejection) | Dense dermal inflammation associated with epidermal involvement (basal keratinocyte vacuolization, keratinocyte apoptosis, and/or necrosis) |
Grade 4 (necrotizing acute rejection) | Frank necrosis of epidermis or other skin structures |
It should be noted here that the above pathological changes are not specific for AR as they can be found in a number of inflammatory, infectious, or proliferative dermatoses [38]. Ancillary techniques have been applied in an attempt to increase the specificity of AR diagnosis, such as immunophenotyping of infiltrating cells or detection of C4d in the allografted skin . The composition of the cell infiltrate is not very discriminative, since it is similar to that found in most inflammatory dermatoses. As discussed above, the presence of FoxP3 + T regulatory cells, detectable in the allograft up to several years post-graft [11, 12] is interesting, although its prognostic significance remains so far unclear.
Chronic Skin Rejection
Surprisingly, despite a high incidence of skin AR, the occurrence of skin chronic rejection (CR) in VCA is rare and has not been reported so far in patients that were compliant with immunosuppressive therapy on long-term follow-up.
Experimental studies have shown that it is possible to induce CR in VCA. Data from a rat hind limb allograft model showed changes consistent with CR after 11 ± 3 episodes of AR. The skin was the main target of the immune response with progressive dermal atrophy and sclerosis and apoptosis of epithelial cells in the hair follicles with permanent hair loss. There are not yet sufficient data available in the clinical setting to define specific changes of CR in VCA. Indeed, the Banff 2007 classification did not include features of CR [33]. Clinicopathological features suggestive of skin CR could include loss of adnexa nail changes, skin atrophy, and fibrosis of deep tissues [33].
We recently investigated all allograft structures by histology , magnetic resonance imaging, ultrasonography, and high-resolution peripheral quantitative computed tomography scan in four bilateral hand transplant patients and one facial allotransplant recipient who all complied with the immunosuppressive treatment. We found no lesions that could suggest CR, such as dermal fibrosis and vascular stenosis [15].
Skin CR has been reported in the first hand transplant recipient after amputation of the graft following discontinuation of his immunosuppressive treatment. The rejected skin allograft showed a histological aspect that resembled chronic lichenoid GVHD [4]. Interestingly, the first patient who lost his hand allograft reportedly because of vascular rejection had no evidence of skin CR at the time of graft loss [6].
Finally, one face transplant patient from our group developed an Epstein–Barr virus (EBV)-positive B cell lymphoma and then a hepatic leiomyosarcoma that necessitated a drastic reduction of the immunosuppressive treatment resulting in multiple AR episodes. The face allograft, as well as the sentinel skin flap, showed some clinical and histological features consistent with CR beginning in the second year after transplantation. This patient showed exactly the predicted lesions of VCA CR, such as a sclerotic aspect of the graft, presence of dense dermal collagen fibers with hyalinosis, and atrophy of the adnexa. The large vessels remained unaffected while the dermal capillaries showed thickened walls and narrowed lumina (unpublished data, manuscript in preparation).
In summary, the experience obtained from 15 years of VCA has shown that chronic skin rejection is a rare event in patients who remain on immunosuppressive treatment. However, it may develop in noncompliant patients or in those where life-threatening side effects necessitate reduction of immunosuppression. One plausible explanation for the discrepancy between the high incidence of acute skin rejection and the absence of chronic skin rejection in compliant patients might be the exceptional healing potential of skin tissue as compared with other transplanted organs (such as the kidney).
Immune Monitoring
Overview on Immune Monitoring in Organ Transplantation
In the past 20 years, major progress has been made in prolonging graft and patient survival after organ transplantation as a result of the development of more efficient immunosuppressive drugs that have dramatically reduced the incidence of acute cellular rejection . However, the occurrence of AMR and subclinical rejection episodes, which markedly influence the long-term graft survival of transplanted organs, cannot be completely prevented with the current standard-of-care immunosuppressive protocols. Furthermore, long-term allograft survival requires lifelong immunosuppression that precipitates renal toxicity, opportunistic infections, diabetes, hypertension, and tumor formation. To avoid the toxic side effects caused by permanent immunosuppressive treatment, research in transplantation has focused on new treatment strategies, such as inducing tolerance or minimizing immunosuppression , in immunologically low-risk patients. The current challenge in transplantation is therefore to develop personalized treatment based on biomarkers at different biological levels that reflect the individual’s immune reactivity and enable transplant clinicians to identify patients at risk for allograft rejection or, conversely, patients in whom immunosuppression could be safely minimized (review in [39]). Until now, monitoring of the immunosuppressive therapy received by solid organ transplant recipients has relied on the measurement of drug blood levels. Although this strategy has shown some efficacy in preventing drug toxicity, it is clearly inaccurate for the evaluation of the level of each patient’s response against his graft, which remains a mandatory step in proposing a tailored immunosuppressive regimen .
Progress in transplant immunology has shown that the recipient’s adaptive immune response relies on two effector arms that reject an allogenic transplant. The cellular cytotoxic response involves graft infiltration by activated T lymphocytes, which induces apoptosis of allogenic cells. The second arm is the humoral response, which relies on the generation of DSA by plasma cells in secondary lymphoid organs. Circulating DSA in turn bind to allogenic targets expressed by graft endothelial cells, which triggers the activation of the classical complement pathway and the recruitment of innate immune effectors responsible for antibody-dependent cell cytotoxicity (ADCC) and microvascular inflammation . Although these two processes usually function in collaboration, some rejection episodes can be exclusively cellular or humorally mediated. Accurate monitoring of allogenic immune responses therefore requires the combination of different techniques.
The question of the monitoring timing is equally important. When performed before transplantation, the tests allow practitioners to grade the a priori “immunological risk” and to adjust the immunosuppression accordingly. Immune monitoring can also be performed as part of the follow-up process posttransplantation, either in regularly predefined intervals or as indicated by graft dysfunction. The former option offers the theoretical possibility to identify recipients at risk for rejection before this becomes clinically apparent. While this strategy can improve long-term outcome, it also requires increased tests (and therefore costs) and defining the time points when the monitoring should be performed.
Another important aspect is the source of the samples available for the monitoring. There is an ongoing debate as to whether the peripheral blood accurately reflects the events occurring within the graft. Although the analysis of a transplant biopsy will undoubtedly give more direct information, sequential analysis, a prerequisite for accurate diagnosis, is unlikely to be possible. For kidney transplant recipients, urine is an easily accessible source of material that has proved to be informative [40]; however, urine biomarkers will not be relevant for patients receiving other types of transplants, particularly vascularized composite tissue allografts.
Finally, it should be noted that immune monitoring in transplantation is an emerging field. Most studies published so far are from single centers and the strength of their conclusions are limited by the inclusion of a small number of patients. Furthermore, most of them focused on renal transplant recipients; therefore, their conclusions may not be directly transposable to the field of VCA. For instance, accumulating evidence points toward a major role for DSA in the immune-mediated failure of kidney, lung, and heart transplants [41]; in contrast, evidence that DSA are capable of driving VCA rejection is still lacking (see above).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








