Fig. 2.1
Commitment of mesodermal stem cells to form different cell lineages in utero. Adipocytes (1), fibroblasts (2), and myoblasts are all derived from parental mesodermal stem cells. The proportion of 1, 2, or 3 may be modified by the in utero environment to which the fetus is exposed. Adipocyte precursor cells may be induced by the interaction of intrinsic agents such as C/EBPα and PPARγ, formation of fibroblasts likely involves the TGF-β signaling pathway, and mesenchymal stem cells receive signals such as Wingless and Int (Wnt) and Sonic hedgehog that regulates expression of Pax3, Pax7, and Gli which initiates the commitment of stems cells to myogenic lineage. In skeletal muscle (the largest tissue of the body) stem cell populations that make skeletal muscle cells, connective tissue proper and adipose tissue develop in close association to each other and may (actually) involve similar maternal stem cells. As such, manipulation of the maternal cell population may determine which of these stem cell populations are established with priority—to the exclusion of the other two. This provides us with a unique system for elucidating the formation, interaction, and developmental progression of these populations of cells. There are perhaps five different myogenic stem cell populations. Some of these populatioins may be detected during different times in utero but others, such as satellite cells, function during postnatal skeletal muscle growth. Adipose precursor cells are defined (mostly) during the initial phases of the third trimester, in utero, and are likely from the same precursor (maternal) population that gave rise to myogenic and fibrogenic precursor cells. Throughout development, intramuscular adipose tissue contains the smallest adipocytes, and adipocyte number increases compared to several other depots. In this regard, adipocyte hyperplasia continues on well after hyperplasia has ceased in most other depots
Differences in development, growth, maturation, and function of subcutaneous, visceral, intermuscular, and intramuscular adipose depots have been documented [1, 4, 6–8, 16–18]. Physiological divergence in adipose tissue depots in terms of potentially regulating other physiology and metabolism of the body has also been defined [1, 16]. Adrenergic innervation density and blood flow in the fasted versus the fed state clearly distinguishes adipose depots [6, 7]. The growth modality (hypertrophy/hyperplasia) and proliferative capacity of both preadipocytes and endothelial cells are depot-dependent traits [6–8]. Depot-dependent differences in adipose tissue metabolism have also been reported [7, 16]. For instance, rates of glucose metabolism and sensitivity to the effect of insulin on glucose metabolism distinguish depots [6], and the basal lipolytic rate and the response to lipolytic hormones are also location dependent [19]. An increased glucose utilization and lipid mobilization, a limited response to hormonal stimulation, and a high blood flow rate may be unique characteristics of a particular adipose depot [1].
Primary (adipogenic) cell cultures have been generated from different adipose depots and include the stromal vascular cell fraction [18] and in a more limited manner the mature (lipid filled) floating cell fraction [20]. Stromal vascular cells are derived from the pellet at the time of cleaning the floating (mature) adipocyte cell fraction during adipocyte isolation [17, 18, 20]. This cell pellet contains a variety of cells that may be inconsistently isolated during different tissue preparations [18], but stromal vascular cells represent one of the most studied of adipogenic cells (Fig. 2.2) [18]. Stromal vascular cells and mature adipocytes have been used for years for many applications to define adipocyte lineage development and metabolism [18]. However, other cells exist within any adipose depot, the use of which might offer elucidation of different mechanisms involved in adipogenesis, lipid metabolism, conversion of cells to other cell types, marker expression, or secretion of regulatory factors which might play a role in regulation of systemic physiology [1, 8, 16–18, 21, 22]. It has been known for a number of years that adipose tissue contains a number of progenitor cells of several cell phenotypes [23]. Morphologically similar to fibroblasts [17], these cells are of potential benefit in the clinical setting [24]. While little is known about potential adipose depot differences in the ability to isolate stem cells, or whether developmental aging influences the cell yield, adipose tissue has been shown to provide a rich source of multipotent stem cells [1, 6, 8, 17, 18, 22–24].
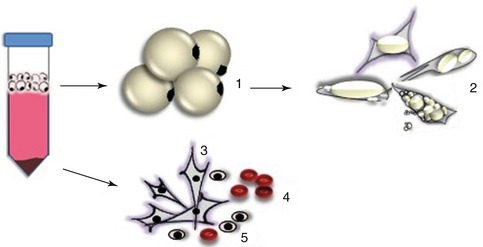

Fig. 2.2
Primary (adipogenic, fibroblastic, and myogenic) cell procurement from postnatal intramuscular tissue; different cells may be isolated as a result of enzymatic digestion of fresh muscle tissue and subsequent differential centrifugation steps. Round mature adipocytes (1) float on the top phase of the isolation tube by means of lipid buoyancy of the cells and are further isolated and cultured with ceiling culture methods (2). All other cells, such as muscle satellite cells and stromal vascular cell fractions, precipitate to the bottom of the isolation tube (3–5). Stromal vascular fraction (alone) is a heterogeneous mixture which contains fibroblast-like cells, which could be preadipocytes, fibroblasts, endothelial cells, white blood cells, macrophage or mesenchymal stem cells, red blood cells, and satellite cells, which depend on the proportion of fat and lean in the original tissue used
Adipocyte precursor cells occupy a bright place in medicine, tissue engineering, and reconstructive surgery [24




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







