Acellular Dermis-Assisted Breast Reconstruction
Scott L. Spear
Pranay M. Parikh
Nathan G. Menon
The Evolution of Prosthetic Breast Reconstruction
Prosthetic breast reconstruction with tissue expanders or implants remains the most commonly used technique for reconstruction after mastectomy, representing more than 70% of all reconstructions performed in 2008 (1). Over the last decade, refinements in mastectomy techniques and device technology and greater coordination between oncologic and reconstructive surgeons have improved the quality of prosthetic breast reconstruction and expanded its role in breast cancer care.
Our techniques for prosthetic reconstruction have also evolved, moving away from total muscle coverage of devices toward subpectoral or “dual-plane” positioning. Advantages of dual-plane device positioning include decreased chest wall morbidity, increased patient comfort during expansion, and shortened operative times (2,3). Limitations of dual-plane device positioning include less secure coverage of the inferior pole of the device by the inferior mastectomy skin flap, less control of the position of the inframammary fold, and superior migration of the pectoralis major muscle during expansion.
Since 2005, Breuing and Warren (4), Salzberg (5), and Zienowicz and Karacaoglu (6), and Spear et al. (7) have each described the use of acellular human dermis as an interposition graft between the chest wall and lateral edge of the pectoralis muscle to realize the benefits of the dual-plane technique while simultaneously overcoming its limitations by using the graft to provide an additional biologic layer of coverage over the inferior pole of the device, to more precisely define the inframammary and lateral mammary folds, and to stabilize the pectoralis major muscle.
Acellular Dermal Matrix
Acellular dermal matrix (ADM) is a biologic material that was introduced in 1994 as a skin substitute for patients with extensive burn injuries (8). ADM is derived from tissue-banked cadaver skin that has been deepithelialized and processed to remove cellular debris and antigenic components triggering immune rejection. The resulting extracellular matrix of collagen, elastin, hyaluronic acid, fibronectin, proteoglycans, and vascular channels provides a scaffold that allows for host cell invasion and repopulation when placed in contact with viable tissue (9). As the matrix is repopulated, the acellular dermis is integrated into local host tissue without immune rejection, chronic inflammatory response, or fibroplasia.
The biomechanical properties of ADM resemble those of normal human dermis, allowing for elasticity, or deformation under load, but also a high peak load to resist tearing (10). These properties make ADM an ideal material for expander-based breast reconstruction, allowing for compliance during expansion while maintaining complete lower pole coverage throughout the reconstruction process.
There are a number of ADM products available on the market, including AlloDerm (LifeCell Corp., Branchburg, NJ), DermaMatrix (Synthes, Inc., West Chester, PA), and NeoForm (Mentor Corp., Santa Barbara, CA). Although each material is based on the concept of rendering cadaveric dermis nonantigenic for implantation, the specific process used by each manufacturer creates variation in the shelf life, preparation instructions, and labeling of each product. In our institution, we have used AlloDerm for all of our acellular human dermis–assisted breast reconstructions since 2004, and the techniques and outcomes presented in this chapter pertain to our experience with this material.
Surgical Technique
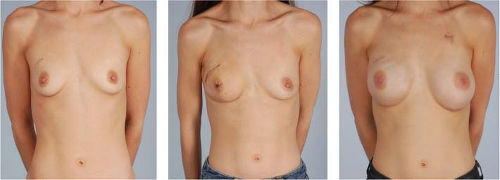
At our institution, we prefer a staged approach to prosthetic breast reconstruction (2,3,7) with immediate placement of a tissue expander and ADM following mastectomy and a delayed exchange to implant following after expansion and completion of adjuvant therapy. Although several authors have achieved good results with one-stage acellular dermis–assisted reconstruction with implants (4,5,6,11,12), we prefer to use an expander initially to minimize tension on the mastectomy flap closure, to ensure skin flap healing, and to facilitate precise device sizing and positioning at the time of exchange.
Stage I
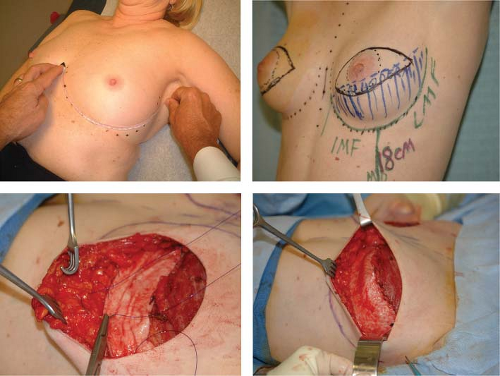
Preoperatively, each patient’s midline, inframammary fold (IMF), lateral mammary fold (LMF), and planned mastectomy skin incisions are marked (Fig. 33.1). Following the mastectomy, skin flaps are assessed for viability, and the IMF and LMF are remarked. For total IMF and LMF arc lengths of 18 cm or less, a 4 × 12 cm, thick ADM graft (AlloDerm; LifeCell Corp.) is selected. For arcs greater than 18 cm, a 4 × 16 cm, thick ADM graft is selected. As reconstruction commences, the ADM graft is reconstituted and rehydrated in normal saline as recommended by the manufacturer.
Next, the pectoralis major muscle is elevated from the underlying chest wall with electrocautery, taking care to leave the pectoralis minor, serratus anterior, and rectus abdominis muscles undisturbed. A small portion of the most inferomedial attachment of the pectoralis major is released as necessary to create the desired pocket shape. Care should be taken not to disturb the sternal origins of the pectoralis to prevent excessive medial migration of the implant or superior displacement of the muscle.
The preoperative markings (IMF and LMF) on the skin surface are then transposed onto the chest wall using methylene blue dye. The reconstituted ADM graft is then placed on the chest wall with the deep dermal surface facing up toward the mastectomy flap. The corners of the graft are anchored to the chest wall at the medial and lateral ends of the proposed IMF and LMF, respectively, using 2-0 polydioxanone sutures. When using interrupted sutures, the central segment of the graft is secured to the proposed IMF and LMF with additional sutures at 1 to 2 cm intervals, leaving the most central sutures untied and clamped with hemostats for later tension adjustments. Alternatively, a running suture technique can be used in which the sutures are not pulled snug until final placement of the expander is completed.
A tissue expander still filled with air, as shipped from the manufacturer, is placed under the pectoralis muscle and the ADM graft. The device is seated medially and inferiorly in the pocket, making sure that the expander’s most inferior edge was all the way down to the new IMF. The inferior border of the pectoralis muscle is precisely aligned to the superior border of the graft with a few staples and then secured with a running a 2-0 polydioxanone suture, closing the pocket over the expander. After securing the pocket, all air is evacuated from the tissue expander. The expander is then instilled with normal saline, and proper seating of the expander is reconfirmed. The repair of the IMF and LMF is completed by tying the remaining untied interrupted sutures or by tightening and tying the lower border running suture. As much as possible, it is important to achieve a “hand in glove,” harmonious fit between the expander, the ADM graft, and the overlying skin flap. As the mastectomy incision is aligned with a few staples, expander filling continues in order to compress the expander against the muscle and ADM graft and to bolster the ADM graft against the mastectomy skin flap. Thus the muscle-graft pocket is made taut, but not so taut
as to exert excessive tension on the muscle-graft pocket closure or skin closure. To reduce the risk of fluid collection around the expander or between the ADM and the mastectomy flaps, a 7-mm flat drain is placed along side the expander, and a smaller 10-French drain is positioned between the ADM and mastectomy skin flap. Final skin closure is completed with a combination of interrupted and running 3-0 poliglecaprone intradermal sutures.
as to exert excessive tension on the muscle-graft pocket closure or skin closure. To reduce the risk of fluid collection around the expander or between the ADM and the mastectomy flaps, a 7-mm flat drain is placed along side the expander, and a smaller 10-French drain is positioned between the ADM and mastectomy skin flap. Final skin closure is completed with a combination of interrupted and running 3-0 poliglecaprone intradermal sutures.
Most patients are discharged on the day following surgery, and oral antibiotics are continued until the drains are removed, typically at 7 to 14 days after surgery. Serial expansion is initiated as soon as the skin incision is healed. Patients are seen at 2- or 3-week intervals until the desired fill volume is achieved.
Stage Ii
Following completion of expansion and adjuvant therapy, patients are scheduled for device exchange. Preoperative planning for the second stage should focus on choosing the most appropriate implant for reconstruction and on identifying any malposition or irregularities to be corrected. Intraoperatively, the prior mastectomy incision is opened, and dissection is continued into the device pocket. The tissue expander is removed, and the capsule is adjusted as necessary to optimize the position of the implant. A saline or silicone implant is then placed into the pocket, and the incision is closed in anatomic layers with polydioxanone sutures in the capsule and intradermal poliglecaprone sutures. When appropriate, nipple reconstruction can be performed simultaneously, and planned symmetry procedures for the opposite breast can also be completed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree