Diagram showing the effect of a closed (a) and an open (b) arterio-venous anastomosis. From Boyd JD. Arterio-venous anastomoses. London Hospital Gazette 1939;42:2–8
42.4 Pathophysiology
The general frostbite sequence begins with complete tissue ischemia, followed by reperfusion, and ultimately tissue necrosis. Manson et al. defined a “double vascular lesion” phenomenon that occurs in cold injury: lack of tissue perfusion from large vessel vasoconstriction and loss microcirculatory control leading to stasis, vessel thrombosis, and tissue ischemia [62]. Three distinct mechanisms are central in understanding the pathophysiology of frostbite by which tissue damage can occur: direct cold-induced cell damage from cell crystallization, indirect cellular injury from local hypoxia from vasoconstriction and microvascular thrombosis, and release of inflammatory mediators post-thaw from reperfusion injury and cell death [15].
Further elucidating the mechanisms are four interconnected pathophysiological phases of the freezing cascade that depend on the temperature, conditions, and duration of cold exposure:
42.4.1 Phase I: Pre-freeze
As tissues begin to cool below 15 °C, vasospasms and eventual vasoconstriction occurs, blood viscosity increases, and tissue perfusion diminishes. Cold-induced vasodilation (CIVD) ceases at temperatures below 10 °C and ice crystal formation begins. This cycling of vasodilation and vasoconstriction is inherently protective to ice crystal formation.
42.4.2 Phase II: Freeze-Thaw Injury
As skin temperature reaches freezing point below −0.5 °C [16, 43], ice crystal formation occurs. There is a distinct difference in pathophysiologic effects with the rate of tissue cooling and absolute temperature to which the tissue is cooled. Rapid freezing of tissue below their freezing point through flash freeze or cold-contact mechanisms leads to the formation of large intracellular and extracellular ice crystals, leading to cell death and irreversible damage to skin [63]. Intracellular ice crystals denature cell membrane lipoproteins and can mechanically disrupt cell membrane integrity. The critical cellular freezing point occurs. With slow freezing, large ice crystals form in the extracellular space, increasing the osmotic pressure and subsequently drawing free water across the cell membrane into the extracellular space which leads to cellular dehydration and interstitial hyperosmolarity as the cell thaws. Cellular dehydration modifies protein structure, alters membrane lipids and cellular pH. Upon rewarming, the intracellular/extracellular ice melts, tissue ischemia is relieved, and reperfusion occurs. As vascular integrity remains grossly intact post-thaw, there is generally full restoration of circulatory reflow with increased vascular permeability due to endothelial damage. With an increase in fluid and protein leakage, blood viscosity further increases and platelet aggregation and coagulation cascade is initiated by the damage to the endothelium basement membrane [64, 65]. As such, despite near initial normal blood flow, within 3–5 min, disruption of flow is seen. Exposure to multiple freeze-thaw-refreeze cycles is detrimental to cell survival [66, 67].
42.4.3 Phase III: Vascular Stasis
As disruption of flow occurs and tissue ischemia persists from vasospasticity and increased blood viscosity secondary to transendothelial plasma leakage, arterio-venous shunting occurs more proximally as distal stasis occurs. These areas of stasis and ischemia lead to the buildup of inflammatory mediators (prostaglandins, histamine, thromboxane, bradykinin) which all propagate progressive tissue ischemia [68]. The combination of stasis and increased viscosity promotes thrombus formation.
42.4.4 Phase IV: Progressive or Late Ischemia
Thrombosis and proximal arterio-venous shunting lead to progressive dermal ischemia and loss of tissue. Final extent of demarcation and tissue necrosis is based most importantly on the degree of microvascular damage and vessel thrombosis. Gangrene eventually occurs from tissue necrosis and depending on degree of vascular compromise, mummification of the tissue can occur in severe cases.
Depending on the time of rewarming and therapeutic management for reperfusion, the increased cellular oxidative stress and inflammation associated with ischemia-reperfusion injury may contribute to further cellular damage and necrosis. The disruption of normal vascular flow due to microvascular thrombosis leads to cellular anaerobic metabolism and subsequent tissue hypoxia. These combined factors further stimulate the increased release of inflammatory mediators, prostaglandins PGF2 and thromboxane A2 (TXA2). Robson and Heggers have reported elevated levels of prostaglandin F2α (PGF2α) and thromboxane B2 (TXB2), an inactive metabolite of TXA2, in frostbite blister fluid. In addition, Özyazgan et al. reported increased prostaglandin I2 and TXB2 in frostbitten tissue by 188% and 249%, respectively. As PGI2 and TXA2 can be seen as physiologic antagonists of one another, it has been postulated that an increase in the ratio of TXA2/PGI2 could lead to increased platelet aggregation and thrombosis and thus the balance between physiologic levels of prostacyclin (prostaglandin I2) and thromboxane A2 is crucial for reducing further tissue necrosis in frostbite injury [69–71].
42.5 Classification
Traditional classification
Degree of severity | Description and presentation | Clinical symptoms |
|---|---|---|
First degree | Superficial partial thickness involvement of the epidermis that is characterized by erythema, edema, hyperemia with possible skin desquamation | Transient burning sensation with throbbing of the area |
Second degree | Full thickness skin freezing that is characterized by erythema, marked edema characterized by vesicles of clear fluid. These blisters may desquamate and eschar formation may occur | Numbness of the affected area |
Third degree | Full thickness skin with subcutaneous tissue involvement that is characterized by violaceous or hemorrhagic blisters with thickened areas of skin necrosis seen as bluish/gray discoloration | No sensation of the area but progresses to shooting burning pain that is throbbing and aching |
Fourth degree | Full thickness skin, subcutaneous tissue, muscle, tendon, bone involvement characterized by little edema with initially mottled deep red or cyanotic area which eventually becomes dry mummified | May complain of joint pain |
Marsigny et al. Clinical Prediction Tool
Frostbite injury of extremities (hands and feet) | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
Extent of initial lesion at day 0 after rapid rewarming | Absence of initial lesion | Initial lesion on distal phalanx | Initial lesion on intermediary and proximal phalanx | Initial lesion on carpal/tarsal |
Bone scanning results at day 2 | Useless | Hypofixation of radiotracer uptake area | Absence of radiotracer uptake area on the digit | Absence of radiotracer uptake area on the carpal/tarsal |
Blister presentation at day 2 | Absence of blisters | Clear blisters | Hemorrhagic blisters on digit | Hemorrhagic blisters over carpal/tarsal |
Prognosis at day 2 | No amputation | Tissue amputation | Bone amputation of digit | Bone amputation of the limb ± systemic involvement ± sepsis |
No sequelae | Fingernail sequelae | Functional sequelae | Functional sequelae |
Wilderness Medical Society Practice Guideline
Degree of severity | Description and presentation |
|---|---|
Superficial | There is none or minimal anticipated tissue loss, corresponding to first- and second-degree injury of the traditional scheme |
Deep | Deeper injury and anticipated tissue loss, corresponding to third- and fourth-degree injury |
Traditional classification follows the classic thermal burn scheme, which is based according to depth of injury, and is defined as follows (Table 42.1):
One criticism of the traditional classification scheme is that it does not take into consideration the unique delayed tissue necrosis demarcation in frostbite injuries and that treatment is directed as a typical burn injury resulting in suboptimal care and patient expectations. As such, the clinical presentation and diagnosis of degree of frostbite injury in a patient who presents acutely in an urgent care setting may not be appropriate, as duration and time of frostbite onset may be unknown.
Of note, it is important to be able to recognize and differentiate “frostnip,” a superficial non-freezing injury of exposed skin, from true frostbite. It is not to be confused with first-degree or superficial frostbite. Frostnip mostly presents as an exposed area with numbness, accompanied by palor or erythema, with potential ice crystal formation on the surface of the skin. By definition, there is no ice crystal formation in the dermis and there is thus no damage done beyond the epidermal layers. What further differentiates frostnip from frostbite is the rapid resolution of symptoms with no long-term sequelae through skin protection and rewarming [72].
Marsigny et al. Clinical Prediction Tool developed in 2001 to classify frostbite injuries of the hands and feet mainly based on locations of lesions and early bone scan results from initial presentation (day 0) (Table 42.2):
Wilderness Medical Society Practice Guideline recommends a simple two-tier classification scheme that can be employed after rewarming but before imaging [72] (Table 42.3):
This system is highly practical and used clinically as most long-term sequelae and tissue viability are unknown until several months from injury. As such, descriptive diagnosis of frostbite degree from the traditional system becomes inaccurate as previously described.
42.5.1 Hennepin Score
Radiography (Limb X-Ray)
Stage and time frame | Radiographic findings |
|---|---|
Early: immediate to weeks after injury | – May be normal, depending on injury severity – Soft tissue swelling – Tissue atrophy and distortion in severely affected areas – Subcutaneous emphysema – No bone or joint changes |
Intermediate: weeks to months after injury | – Bone demineralization – Periostitis |
Late: months to years after injury | – Acro-osteolysis – Sclerosis at ends of involved bone – Asymmetric early osteoarthrosis of the affected limb – Small periartibular erosion – In children, epiphyseal fragmentation and/or premature fusion with resulting deformities |
The purpose of the Hennepin score is to devise a standardized rating scale for researchers across academic centers to accurately measure injury and salvage rate outcomes to evaluate treatment efficacy. Imaging modalities that are included in the scale are Doppler ultrasound, magnetic resonance imaging, angiography, and bone scanning [61]. However as we will discuss below, currently there are no accurate means for diagnosis at the early stages of frostbite.
42.6 Diagnostic Methods
Most frostbite injuries are diagnosed clinically in the context of symptoms, physical examination, detailed history. In the setting to determine the extent of soft tissue injury and long-term tissue viability, diagnostic radiologic imaging modalities that aim to determine tissue perfusion and vessel patency have proven valuable. The main objective of imaging in the context of frostbite is to assess depth of involvement, severity, and to direct treatment based on surgical or non-surgical indications. It also allows for objective determination of frostbite treatment response and efficacy.
42.6.1 Radiography (Limb X-Ray)
In general, X-ray is not useful in the initial context except to rule out a trauma-related fracture. It is however, a rapid and inexpensive imaging modality in the late context that can show bone demineralization changes as soon as 1 week after frostbite injury, and bone artifacts and/or epiphyseal arrest after 6 weeks of injury in children [12]. Millet et al. have described the radiographic findings in relation to the stages of frostbite.
In summary, these radiographic findings can range from a normal X-ray with evidence of soft tissue swelling to severe bone destruction and demineralization depending on the severity of frostbite and the duration of time since the injury. Early radiographic evidence of mild injury can demonstrate osteopenia or show no prominent pathology. However most notable is the evidence of acro-osteolysis, sclerotic areas at terminal ends of affected bones, as well as early osteoarthritis, months to years after injury. All of which are indicative of previous deep frostbite injury that involve the tips of fingers or joints [73]. In children, epiphyseal fractures and premature fusion of the growth plates have been reported to occur, often months to years after injury. This can result in finger malformation and debilitating chronic joint problems. As a clinical tool, radiography can be a useful modality to follow the progression of injury but there may be discrepancies in clinical correlation as radiographic evidence is nonspecific and has no predictive value in determining the level of tissue necrosis. Management would indeed be based on clinical examination.
42.6.2 Digital Subtraction Angiography (DSA)
The main goal of using DSA is to identify potential targets for thrombolysis in patients presenting within 24 h with deep frostbite injury. Initial DSA will demonstrate lack of perfusion in affected digit and may show areas of impaired perfusion that do not appear affected on physical examination. Patients undergoing thrombolytic therapy can be followed with repeat DSA imaging at 12 h increments for up to 48 h to assess response to thrombolytics. By reversing the microvascular thrombosis present in frostbite injury, flow can be restored and prevent further tissue ischemia [74, 75].
42.6.3 Magnetic Resonance Imaging
Magnetic resonance angiography (MRA) has been suggested as a noninvasive alternative to DSA for evaluating the patency of vessels in frostbite injuries [76]. However, this modality lacks the benefit of being both diagnostic and therapeutic, unlike DSA. There is limited evidence suggesting that MRA might be able to define occluded vessels and demarcate soft tissue injury after more than 24 h of injury [77, 78].
42.6.4 Technetium (Tc)-99 m Scintigraphy
Also known as triple-phase bone scanning, Technetium-99 m (Tc-99m) has been in use for the past two decades for evaluating frostbite wounds. It involves a nuclear isotope that is taken up by osteoblasts. If the bone’s blood supply has been compromised secondary to frostbite, the tracer will not be present in the bone. There are 3 phases in scanning: flow phase, blood pool image, and delayed phase. The first phase (seconds after the injection of the isotope) illustrates perfusion to an area. The blood pool phase occurs 5 min after injection and this shows the vascularity of the region. Finally, the delayed phase occurs about 3 h after injection. By this time, most of the isotope will have been metabolized and bone turnover can be better assessed.
Tc-99m scintigraphy is indicated in patients who present with deep (second, third, and fourth degree) frostbite injuries and is recommended to have bone scanning performed within 2–4 days after frostbite injury [79, 80]. The scan should not be performed immediately after cold exposure, as microvascular thrombosis can progress over time and what is defined on imaging may not be the level of tissue necrosis. Cauchy et al. in 2000, report that the level of amputation can be closely predicted in approximately 84% of cases at the initial scan, many weeks before the nonviable tissue declares itself on physical exam. Moreover, it has been suggested that any blisters should be debrided before the scans are performed to prevent accumulation of tracer in the blister fluid and lead to false-positive interpretation [80]. Again, larger prospective randomized studies are warranted to evaluate the reliability of such an imaging modality to predict the level of tissue demarcation and subsequent amputation, which would ultimately limit patient morbidity.
42.6.5 Single-Photon Emission Computed Tomography + CT (SPECT/CT)
By combining both the functional information from scintigraphy (bone perfusion) and uptake with the anatomic information derived from CT, a more specific image can be rendered than a conventional bone scan alone. More specifically, a CT scan is sequentially performed immediately after the delayed phase of nuclear bone scan and the images are merged, allowing for more exact delineation of the level at which the bone loses perfusion. It becomes particularly useful in assessment of the distal ends of digits as conventionally these regions can be difficult to properly visualize on bone scintigraphy alone. Most recently in a retrospective case series (N = 7), Kraft et al. describe the effectiveness of SPECT-CT in determining level of more distal amputation, allowing for preservation of digit length [81]. Six patients were able to undergo more distal amputation based on SPECT-CT imaging correlation. Although suggesting that SPECT-CT has a favorable predictive capacity, this is early evidence and further comparative and prospective studies are warranted for validation. It may be an important modality for surgical planning and minimizing the amount of tissue that is excised and limit patient morbidity.
42.6.6 Microangiography
Recently, Masters et al. describe a case report indocyanine green fluorescence microangiography to monitor clinical progression of perfusion in severe frostbite in hyperbaric oxygen therapy and propose its potential role in frostbite monitoring [82]. The benefits of indocyanine green microangiography are that it can be administered through a peripheral intravenous line; it is hepatically cleared and is thus safe in renally impaired patients and has a short half-life. On a technical and operator standpoint, it also does not require the consultation of a radiologist or dedicated imaging department, but rather can be done in the office or clinic. The dye travels to areas where there is perfusion and with a near-infrared laser and camera, blood flow is visualized by brightness. Given the potential benefits and portability of this imaging modality, further studies are required to determine its efficacy and practicality in the setting of frostbite.
42.7 Management
As initially indicated, the key factor that will determine the type of management is duration of exposure to subzero temperatures. The classic management of frostbite has been resuscitation, rewarming, and watchful waiting. Over the past 50 years, the adage “Frostbite in January, amputation in July” remains relevant despite advancements in the understanding of frostbite pathogenesis and advancements in thrombolytic therapy. The main goal of treatment is to prevent further tissue damage and to limit limb morbidity. As such, rapid triage and initiation of proper treatment for frostbite can lead to remarkable improvements in outcome and prognosis.
42.7.1 Clinical
The initial clinical manifestations of frostbite injury are similar for superficial and deep tissue damage, thus early treatment is identical for all injuries.
42.7.1.1 Rewarming
The mainstay of treatment is to ensure that core body temperature is raised to near physiologic 37 °C and that rewarming of the affected area is quickly initiated. Rapid rewarming ideally occurs through total immersion of the affected area in a warm whirlpool water bath between 37 and 44 °C [15]. Given that it has been shown that anoxic reperfusion injury occurs from slow thawing, rapid rewarming is recommended [15, 63, 83]. Rewarming time can vary from 15 to 30 min and up to an hour and can be stopped based on clinical judgment of tissue color with the goal of a red/purple color and good tissue pliability [29, 72].
42.7.1.2 Blister Debridement
Rewarming of skin in cases of superficial frostbite may result in the formation of clear blisters while cases of deep frostbite results in hemorrhagic blisters [2]. It has been shown that blisters filled with clear or milky fluid contain elevated levels of inflammatory mediators prostaglandin F2α (PGF2α) and thromboxane B2, an inactive metabolite/product of thromboxane A2 (TXA2), which both propagate platelet aggregation, thrombosis, and vasoconstriction. As such, in order to prevent further damage to the sub-dermal plexus, most evidence in literature supports superficial debridement of white or clear blisters. It is however not recommended to debride blisters in the field to prevent infection [84]. It is also an indication to debride blisters if they are on joint surfaces and restrict movement [85]. Should blisters be debrided, the wound is to be covered with topical antimicrobial and possibly aloe cream, which has properties that inhibit the arachidonic acid cascade and thromboxane synthesis [15]. There is no evidence supporting either debriding or leaving intact hemorrhagic blisters.
42.7.1.3 Tetanus Prophylaxis
The administration of tetanus toxoid is based on standard guidelines. Frostbitten tissues are not especially prone to tetanus infection [29, 86].
42.7.1.4 Systemic Antibiotics
The call for systemic antibiotic administration is based on the presence of infection or open trauma. The role and benefits of prophylactic antibiotics in frostbite has not been proven and is not recommended unless signs of infection develop [29, 87, 88]. Antibiotics should be considered for prophylactic administration in severe frostbite injuries (second or third degree) where there is presence of an open wound [84, 88].
42.7.1.5 Wound Care
Unsalvageable tissue will eventually necrose and potentially become gangrenous without proper wound care. Tissue gangrene and mummification requires daily wound care to ensure that the wound stays dry to prevent wet gangrene infection. Use of topical antimicrobial dressings similar to burn dressings are recommended until mummification occurs after which dry dressings can be used. Furthermore, tissue protection through removable protective splinting, interdigit padding, or orthotics is also important considerations during the demarcation period to prevent further tissue tear and infection [29].
42.7.2 Therapeutic
As of current literature, there are no human randomized controlled trials with an objective reproducible method to assess the change in demarcation level from the intervention, making the recommendation of therapeutic interventions difficult. There are several emerging treatment options being increasingly studied in cases of severe frostbite within the first 24 h of injury.
42.7.2.1 Topical Aloe Vera
As elevated levels of prostaglandin production contribute to the pathogenesis of frostbite, early prevention with anti-thromboxanes such as topical aloe vera gel has suggested as treatment adjuncts. Aloe vera has been shown to inhibit TXA2 synthetase and maintain PGE2 and PGF2α levels to maintain vasodilation in both thermal and frostbite injuries [89, 90]. An early animal study using frostbitten rabbit ear models showed that tissue survival can be improved with the administration of topical aloe vera and that its effects are comparable to the therapeutic effects of systemic pentoxifylline, a phosphodiesterase inhibitor [91]. Clinically, it is to be applied to all frostbitten areas every 6 h until wound healing is completed.
42.7.2.2 Non-steroidal Anti-inflammatory (NSAID) Medication
Most commonly used NSAIDs are ibuprofen and aspirin (ASA) which hold the dual purpose of providing anti-inflammatory activity and analgesia. This medication work by inhibiting cyclooxygenase enzymes (COX) that converts arachidonic acid to prostaglandin H2 (PGH2), which is ultimately converted to other prostaglandins (PGD2, PGE, PGF2, PGI2) involved in inflammation, as well as TXA2. Ibuprofen is a nonspecific COX inhibitor, reversibly blocking both COX-1 and COX-2, but has higher inhibition of thromboxanes than other prostanoids [87, 92]. Early oral administration of ibuprofen at a dose of 12 mg/kg/d to a maximum of 2400 mg/d provides early systemic anti-prostaglandin activity, limiting inflammatory damage [86]. Similarly, aspirin is also an effective analgesic and suppresses prostaglandins and thromboxanes through irreversible inactivation of COX-1 and COX-2, thus having a prolonged anti-platelet property. ASA may also inhibit endothelial cell synthesis of PGI2, a prostaglandin involved in platelet aggregation inhibition. Although, in terms of pathophysiology, the use of either aspirin or ibuprofen in initial supportive frostbite treatment would be reasonable, there is insufficient evidence to support the benefits of aspirin in preventing tissue loss secondary to frostbite. The only study in humans supporting aspirin dates to 1983, whereby 38 patients with first- and second-degree frostbite upon presentation were treated with ASA and aloe vera showing no major tissue loss. However, there was no control group, 2 patients with acute second-degree progressed to third degree, and no mention of the time to treatment post-injury [68]. Conversely, ibuprofen has shown stronger evidence to support its efficacy in frostbite. A nonrandomized control trial by Heggers et al. in 1987 reported that patients treated with ibuprofen and aloe vera had a significant reduction in morbidity, whereby in all degrees of frostbite 67.9% healed without tissue loss vs. 32.7% in the control group, and 7% in the ibuprofen treatment group vs. 32.7% in the control group required amputation. As such, evidence thus far has suggested that ibuprofen should be considered as adjuvant therapy for the management of frostbite [12, 29, 93]. There remains no study that compare directly aspirin and NSAID for frostbite treatment, and there is no study that compares the different types of anti-inflammatory agents in frostbite therapy.
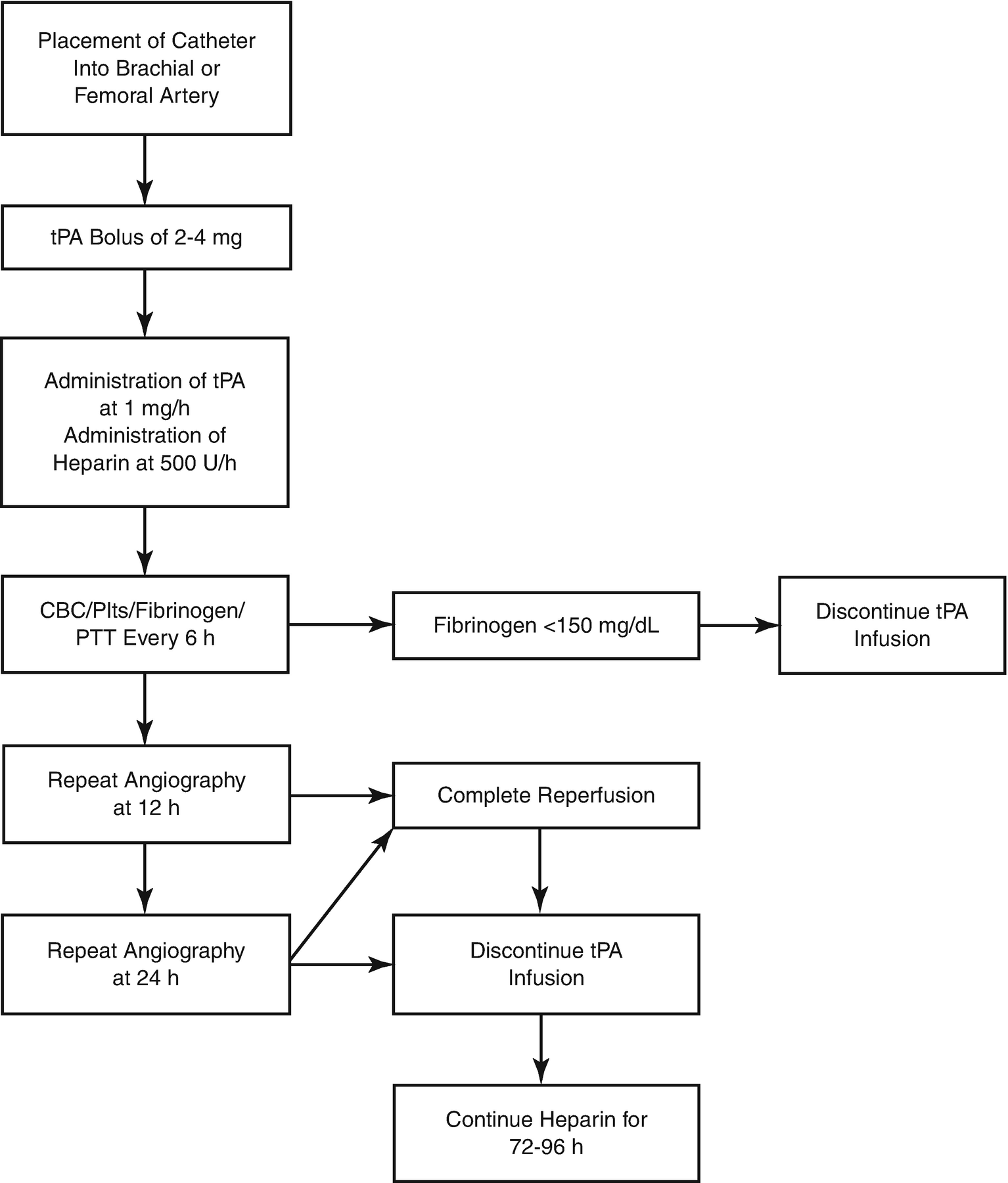
42.7.2.3 Tissue Plasminogen Activator (tPA)
A protease enzyme involved in fibrinolytic pathway in the breakdown of blood clots, tPA has been used as a mainstay treatment in the setting of acute ischemic stroke and is postulated to resolve microvascular thrombosis and ultimately restore perfusion in severe frostbite injury. The mechanism of thrombolysis is through the activation of plasminogen conversion to plasmin, which is capable of cleaving cross-links between fibrin molecules that form thrombi. Historically, evidence to support the effectiveness of thrombolytics in frostbite dates back to the 1980s where antithrombotic agents (streptokinase, urokinase) were proposed in rabbit and rat animal models, showing positive results [94, 95]. The earliest clinical data of tPA use in frostbite was in 1992, whereby 14 patients with severe frostbite, confirmed through triple-phase bone scanning, were treated conservatively with supportive measures (N = 10) and tPA (N = 4). It was seen that all 10 patients treated with supportive therapy required amputations while 3 out of 4 (75%) of those treated with tPA required no amputation [65].
More recently, two studies have reported significant limb salvage rates in severe frostbites following tissue plasminogen activator (tPA) therapy. In 2005, Twomey et al. published a nonrandomized prospective trial with historical controls, reporting an 18.9% rate of amputation in 19 patients with severe frostbite with a total of 174 digits at risk, 33 of which required amputation, that were treated with intravenous tPA (N = 13), intra-arterial tPA (N = 6), and subsequent intravenous heparin. Those who did not respond to therapy were more than 24 h post-injury, warm ischemia time of greater than 6 h, or evidence of multiple freeze-thaw cycles [74]. Furthermore, this study supported that tPA and heparin administration concurrently are safe and tPA administration, whether intravenous or intra-arterial, shows similar therapeutic effect in terms of digital limb salvage. In 2007, Bruen et al. published results from a single-center, retrospective review, comparing 32 patients recruited from 2001 to 2007 who were treated with intra-arterial tPA to historical controls from 1995 to 2001 presenting more than 24 h post-injury. It was found that those with severe frostbite treated with tPA within 24 h of injury and found the incidence of digital amputation was reduced from a potential amputation rate of 97 out of 234 digits (40%) at risk to 6 out of 59 digits (10%) at risk [75]. Similar to Twomey et al., it was noted that the patients who failed to improve blood flow and required amputations were those who presented to hospital post 24 h from injury. Both studies suggested that early tPA administration can result in digital salvage rate of 85–90% [75]. However, both studies use nonrandomized controls without an objective means of assessing the demarcation level.
In regard to thrombolytic therapy in general, Gonzaga et al. in 2016 published a retrospective observational cohort study of 69 patients from 1994 to 2007 with severe frostbite confirmed by angiography, 62 of whom underwent thrombolytic therapy which included Urokinase (N = 19), tPA (N = 18), Reteplase (N = 14), and TNKase (N = 11). Of these groups, there was no significant difference in response to different thrombolytic agents. They report a combined 68.6% digit salvage rate with 148 digits requiring amputation out of 472 digits at risk. Similar to both studies by Twomey and Bruen, Gonzaga et al. also describe the scenario in which 7 patients were given intra-arterial thrombolytic therapy post-24 h and none of the patients responded, all requiring digit amputations [63].

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








