38 Brachial Plexus Palsies
Summary
Pediatric brachial plexus palsies can present at birth (1 in 1000 newborns) and from blunt trauma in older children. The extent of involvement and severity of injury is variable. This chapter will discuss assessment, nonsurgical treatment, and surgical treatment of brachial plexus palsy.
The approach to surgical exploration will be detailed and a spectrum of scenarios will be presented so that the principles of primary nerve reconstruction (including nerve graft and nerve transfers) can be illustrated. These include upper plexus, pan- plexus, multiple root avulsions, isolated deficits, delayed presentation and failed reconstruction. Technical details of nerve grafting and nerve transfers will be described.
Secondary musculoskeletal consequences of brachial plexus palsy will also be discussed including strategies for prevention and options for secondary surgical reconstruction.
38.1 Introduction
The brachial plexus is a complex network of nerves that provide sensation and motor control of the upper extremity. In addition to the intricacies of its form and functions, growth and development in the setting of chronic denervation add another layer of complexity to the management of pediatric brachial plexus palsies.
An understanding of the anatomy and presentations is important in the evaluation of patients. An appreciation of the natural history and outcomes is critical when deciding upon treatment. Preservation of musculoskeletal health in the setting of chronic denervation is needed to optimize functional results.
Given the many dimensions of pediatric brachial plexus palsy, children are best served through a multidisciplinary team approach at specialized centers offering coordinated, longitudinal care. Early referral allows for better clinical assessment and may reduce the chances of sequelae that affect long-term outcomes.
38.2 Presentation
Neonatal brachial plexus palsy (NBPP) occurs in 1/1,000 live births and presents immediately after delivery. Downward traction on the brachial plexus results in injuries of the upper plexus. With further traction, the zone of injury progresses to lower regions of the plexus, ultimately resulting in pan-plexus palsy in its most severe expression. Presentations can generally be grouped into four patterns: type I involves C5 and C6 deficits (Erb/Duchenne type) with loss of shoulder abduction, shoulder external rotation, elbow flexion, and forearm supination. The limb assumes the Erb posture due to intact antagonist muscles. Type II involves C5–C7/C8 deficits (extended Erb type), resulting in the additional loss of wrist extension. The limb assumes the “waiter’s tip” posture. Type III involves deficits from C5 to C8/T1, resulting in an arm that is generally flaccid or paralyzed. Type IV involves C5 to T1 as well as the sympathetic chain, resulting in flail arm with a Horner syndrome.
Upward traction on the brachial plexus results in injuries of the lower plexus, resulting in loss of hand and wrist function with intact elbow and shoulder function. This presentation is known as Klumpke’s palsy and is extremely rare in NBPP.
When a root level is affected, the site of injury tends to be at the trunk level in the upper plexus and more proximal in the lower plexus. This may be related to the presence of fascial sleeves that extend from the bony vertebrae to the extraforaminal nerve roots in the upper plexus. This fascia is progressively absent at more inferior nerve root levels. As such, lesions of the lower roots are more likely to be preganglionic spinal root avulsions. One exception to this pattern occurs in breech deliveries, in which C5 and C6 lesions tend to be avulsions.
Traumatic brachial plexus palsy (TBPP) can occur in older children, but is rare. The mechanism is usually blunt injury and most often results from motorized vehicle accidents. Compared to NBPP, the forces involved are rapid and high energy. Consequently, the patterns of injury in TBPP are varied and more likely involve spinal root avulsions.
Injuries associated with NBPP can include fractures of clavicle, humerus, and rarely cervical spine. Phrenic nerve injury can occur with severe palsies, and hypoxic brain injury can result from asphyxia. Children with TBPP should undergo a general trauma survey.
Several conditions may present with altered limb posture or movement and must be differentiated from brachial plexus palsy. “Pseudoparalysis” can result from pain related to bony fracture; however, once healed, normal limb function resumes. A septic shoulder may present with loss of shoulder movement, but fever, constitutional symptoms, and a “hot joint” will often be present. Congenital limb differences such as arthrogryposis can be distinguished by skeletal hypoplasia, absent skin creases across joints, and associated anomalies. Lesions of the central nervous system may present with altered tone, lower extremity findings, global cognitive impairment, and/or progressive weakness.
38.3 Assessment
38.3.1 Initial Assessment
Initial history focuses on birth, associated injuries, limb presentation, and progression of recovery. Specific limb postures should be elicited, given many families will describe a flaccid arm in spite of intact motor functions. For example, when hand function is present, it can be helpful to understand if the hand function has always been present (i.e., a Type 1 palsy) or if that function has recovered (i.e., a Type 3 palsy with persistent upper plexus deficits). Any history of recovery helps understand the type of nerve injury that has occurred. Neuropraxia (Sunderland 1) involves no Wallerian degeneration, and function recovers rapidly. Axonotmesis (Sunderland 2) involves Wallerian degeneration and requires axon regrowth. In general, recovery of full active range of motion within 1 month of age is associated with no measurable long-term deficits in strength, sensation, or limb growth. Slower recovery (i.e., Sunderland 3 and 4 injuries) is associated with greater long-term deficits. Complete nerve disruption (Sunderland 5) results in no recovery.
Initial examination includes an assessment of global cortical function including age-appropriate developmental milestones, trunk control, tone, and posturing. Neurologic examination of lower extremities helps rule out central nervous system lesions. Respiratory function should be assessed to screen for associated phrenic nerve injury. The face should be assessed for Horner syndrome (ptosis, myosis, anhydrosis) that results from disruption of the sympathetic chain. Given that the most cephalic contribution to the sympathetic chain exits the spinal cord along the T1 foramen, persistent Horner syndrome associated with pan plexus palsy suggests a proximal T1 injury and possibly an avulsion. Scapular stability and rhomboid function should be assessed to determine involvement of dorsal scapular nerve. Given the very proximal divergence of this nerve from the brachial plexus, a deficit suggests a proximal zone of injury that may involve a preganglionic root avulsion. Infants with NBPP should also be assessed for torticollis, which is frequently present and may be related to traction injury to neck musculature.
Focused examination of the brachial plexus follows the more general initial exam. Examination of infants with NBPP is limited to observed and encouraged movements and these can be rated according to the Active Movement Scale (AMS; Table 38‑1). Movement at each major limb articulation is examined and recorded at each visit. Infants may or may not “perform” on a given day and repeated examination helps in developing a more reliable assessment.
Score | Gravity | Observation |
0 | Gravity eliminated | No contraction |
1 | Gravity eliminated | Contraction, no motion |
2 | Gravity eliminated | ≤1/2 active range of motion |
3 | Gravity eliminated | >1/2 active range of motion |
4 | Gravity eliminated | Full active range of motion |
5 | Against gravity | ≤1/2 active range of motion |
6 | Against gravity | >1/2 active range of motion |
7 | Against gravity | Full active range of motion |
Older cooperative children with TBPP may undergo more detailed examination including sensation and specific muscle group testing. Both AMS and British Medical Research Council (MRC) scale can be used to record motor function. The Mallet scale measures composite motions that are important for activities of daily life, and is useful for assessing outcomes and for determining the need for secondary procedures in children with both NBPP and TBPP (Fig. 38‑1).

One of the difficulties in management of nerve injuries is the uncertainty of a single examination. The spectrum of the extent of injury (i.e., zone of injury), severity (i.e., Sunderland class), and the fact that variations in injury severity can exist in different regions of a patient’s brachial plexus make the prediction of outcome complex. In infants with NBPP, electromyogram (EMG) has been found to be “overly optimistic.” As such, an appreciation of the severity of nerve injury relies upon repeated longitudinal assessments.
Early assessment within 1 month of age or injury establishes a baseline, and repeated monthly or bimonthly examination helps understand the pattern of recovery. Assessment by a consistent examiner helps reduce variation. Ongoing therapy and nonsurgical treatment are critical and should be incorporated into assessments and longitudinal care.
38.4 Nonsurgical Treatment, Natural History, and Indications for Nerve Surgery
38.4.1 Nonsurgical Treatment
All children with brachial plexus palsy should receive nonsurgical treatment with therapy to maintain passive range of motion, encourage limb awareness, maintain sensory feedback, and facilitate cortical development and functional adaptation. Dedicated physical and occupational therapists, as well as rehabilitation specialists with expertise in pediatric brachial plexus palsy are central in these efforts. Nonsurgical treatment may be more important to outcomes than surgical treatment, given that contractures and limb neglect can negate any improvements from nerve reconstruction. More detailed discussions of strategies to avoid musculoskeletal complications follow (see section “Musculoskeletal considerations and complications”).
The decision to pursue nerve reconstruction is based on the fundamental question of whether the results with surgery will be better than nonsurgical treatment alone. Given that nerve reconstruction is imperfect and final outcomes are uncertain, an understanding of natural history and surgical outcomes is important in deciding a course of treatment.
38.4.2 Indications for Surgical Exploration: Neonatal Brachial Plexus Palsy
The majority (70–90%) of infants with NBPP rapidly recover adequate function that nerve exploration is not indicated.
Most agree that a flail arm (i.e., flaccid limb with no hand function) associated with a persistent Horner syndrome beyond 1 month of age portends to a poor prognosis. The association of Horner syndrome with no hand function suggests a proximal injury of T1 (i.e., nerve root avulsion). There is a consensus that in this situation, early exploration and reconstruction are warranted in attempt to achieve reinnervation of hand functions.
Tassin (1983) studied a cohort of children with NBPP and found that infants who did not recover any elbow flexion by 3 months tended to have poor long-term shoulder and elbow function. Based on that study, Gilbert recommended surgical exploration for any infant who did not recover biceps by 3 months of age. Although Gilbert’s reported outcomes are favorable, these have not been compared to outcomes without surgery.
Both Carter (2004) and Clarke (2007) have suggested that absence of elbow flexion at 3 months may be an overly aggressive indication for surgical treatment. Waters (1999) compared the results of microsurgical reconstruction with outcomes of nonsurgical treatment and stratified patients by the month after which elbow flexion recovered. He found that when elbow flexion takes greater than 5 months to recover, outcomes are better with surgery. He recommends the absence of elbow flexion within the first 6 months of life as an indication for nerve reconstruction.
Surgical indications based on the absence of elbow flexion or “biceps function” simplify recovery of the brachial plexus to a single clinical finding. Whereas simplifying decision-making for a complex condition has merit, our preferred approach considers all of the movements of the limb.
Clarke (1994) examined a cohort of patients with NBPP treated nonsurgically and analyzed all limb active movements at 3 months of age using the Toronto scale. Although lack of elbow flexion was the most significant predictor of a poor outcome, the lack of wrist, finger, thumb, and elbow extensions were also independent predictors of poor outcome. These findings led to a statistical model in which a composite AMS score for these movements (elbow flexion, elbow extension, wrist extension, thumb extension, and finger extension) of less than 3.5/10 always predicted a poor outcome, and was thus an indication for surgical treatment. The clinical picture of this score is that of C5–C8 or worse deficits (i.e., pan-plexus or near pan-plexus palsy) at 3 months of age. These indications did not account for all patients with a poor outcome. As such, an additional test at 9 months was added for those infants who had adequate scores at 3 months of age. If a child was unable to pass the “cookie test” (the composite movement of getting hand to mouth with arm in an adducted position), they were offered surgical treatment. Fifteen years later, Clarke published results of nerve reconstruction (2009) suggesting that the results of nerve grafting were better than neurolysis (and presumably no treatment) based on the above indications for surgery. The Toronto protocol has evolved to include the lack of elbow flexion at 6 months as another indication for surgical treatment (Table 38‑2).
38.4.3 Indications for Surgical Exploration: Traumatic Brachial Plexus Palsy
Older children with TBPP need to be considered on an individual basis given that there is little data to guide generalized treatment. The rapid, high-energy nature of TBPP results in greater variations of presentation and a greater likelihood of root avulsions. Older children have less capacity for nerve regeneration and the distance for axons to reach target is greater. In addition, development is not as rapid and cortical plasticity may not be as great. Principles applied to adult brachial plexus palsy may be adapted to the management of children with TBPP.
The presence of a Tinel’s sign and careful clinical examination can help localize the specific site of injury. Given that axons grow at a rate of 1 mm per day, the measured distance from site of injury to target muscle can be used as a guide to determine how long an axonotmetic injury would be expected to recover. Exploration is considered if there is no evidence of nerve recovery beyond this time. As opposed to infants, serial EMG provides insights into potential recovery and should be used as an adjunct to clinical exam.
38.4.4 Investigations Prior to Nerve Exploration and Reconstruction
Once the decision to proceed with surgery has been made, assessment for nerve root avulsion can help with surgical planning. Nerve root avulsions are preganglionic lesions in which discontinuity of neurons from the spinal cord renders the root nonfunctional and without the possibility of functional end target reinnervation. A pseudomeningocele with absent rootlets is highly predictive of an avulsion. Computed tomography (CT) myelogram has been the standard imaging modality; however, this requires a lumber puncture and significant radiation exposure in a young infant. We have compared newer techniques of magnetic resonance (MR) myelography and have found them to be equal in accuracy to CT myelogram (Tse 2014). MR myelography does not require lumbar puncture and avoids radiation exposure. Another advantage of MR is that bilateral shoulders can be included in the study to assess for glenohumeral dysplasia (GHD), skeletal changes that occur with chronic muscular imbalance. We have examined scans of infant shoulders and have found a very high incidence of skeletal changes (74%) that can occur early and in the absence of limited passive range of motion. If significant skeletal changes are detected, the method of postoperative immobilization can be modified to address these abnormalities.
If nerve transfers are anticipated, EMG can be used to confirm suitability of potential donor nerves for transfer, given that some donor nerves can be affected by the brachial plexus palsy. For example, involvement of C7 results in partial denervation of the triceps muscles and can affect results of radial to axillary nerve transfers. EMG is used to assess the status of potential donors and to select the best one.
Ultrasound of the diaphragm is routinely performed to assess the functional status of the phrenic nerve, which can be intertwined with injured nerve elements and result in limited respiratory reserve.
38.5 Nerve Exploration and Reconstruction
38.5.1 Positioning
The posterior triangle of the neck can be accessed in the supine position by turning the head to the opposite shoulder (neck rotation and flexion; Fig. 38‑2). A midline bump supports the kyphotic curvature of the lower cervical and upper thoracic spine to bring the plane of the brachial plexus parallel to the floor. The arm, shoulder, neck, and chest on the affected side should be exposed. Dissection is carried out with shoulder adducted to maximize the costoclavicular space.

38.5.2 Exposure
Supraclavicular Region
A horizontal incision is designed along a skin crease parallel to the clavicle, extending from the clavicular insertion of the sternocleidomastoid to the medial insertion of the trapezius. A vertical limb parallels the posterior border of the sternocleidomastoid and extends approximately midway to the mastoid (Fig. 38‑2). Although a single horizontal incision can be used, the exposure is more limited and relies upon soft-tissue retraction.
The initial incision is made through platysma, and the superolaterally based flap is elevated in a subplatysmal plane. The playsma is thin and difficult to visualize in infants, but can be differentiated from overlying subcutaneous fat and underlying supraclavicular fat pad by its white smooth texture (Fig. 38‑3). The external jugular vein and the cervical plexus of sensory nerves are deep to the platysma and can also help define the appropriate plane of dissection. The external jugular vein needs to be retracted or ligated and the cervical plexus should be lysed from the network of veins intertwined with it so that it can be later used for transfer or as graft material (it is easier to clean the nerves before dividing distally). Each of the cervical plexus branches is traced proximally where they converge with each other and with the great auricular nerve (which courses over the anterior surface of sternocleidomastoid; Fig. 38‑4 a). Further dissection proximal reveals the C4 root and the phrenic nerve in a region outside of the zone of injury and severe scar (Fig. 38‑4 b).


The C4 contribution to phrenic nerve is usually the most medial branch and can be found by gentle blunt dissection over the anterior surface of the anterior scalene. It takes a direct caudal course, paralleling the fibers of anterior scalene, and is under the prevertebral fascia. Its identity can be confirmed by electrical stimulation.
Once the phrenic nerve has been visualized, the supraclavicular fat pad is divided along the posterior border of the sternocleidomastoid and superior border of the clavicle. Bipolar cautery is used to reduce the possibility of lymphatic leak. Traction on the fat pad should be avoided, given this layer is continuous with the tissues that envelop the carotid sheath (whose contents can become unnecessarily exposed). Care should also be taken deep to the junction of sternocleidomastoid muscle and clavicle to avoid inadvertent injury to the thoracic duct or exposure of the subclavian vein. The bluish hue of the latter can sometimes be seen within the fat if the external jugular vein is followed too far caudal along its course. Clean incisions and preservation of the supraclavicular fat pad facilitates later repair, which is important in providing a protective layer for nerve reconstruction, an ideal gliding surface for nerve excursion, and a vascular environment for nerve grafts.
Deep to the fat pad, the transverse cervical artery and vein can usually be found running parallel to the clavicle. These vessels are often ligated for exposure. The omohyoid muscle is encountered at its tendinous midportion before exposing the brachial plexus. This muscle can be retracted or transected, and should be mobilized adequately to allow transposition so that it lies superficial to the supraclavicular fat pad at closure. This avoids muscle movements directly over newly reconstructed nerves.
The upper trunk of the brachial plexus is found just deep to the omohyoid muscle, and is often heavily scarred and distorted (Fig. 38‑5). Following the principle of working from “known to unknown,” the brachial plexus is exposed and defined starting outside of the zone of injury to facilitate the more difficult dissection through the scarred region.

The previously identified phrenic nerve can be followed along its caudal course from the C4 root on the anterior surface of the anterior scalene. As the nerve approaches the lateral border of muscle, it gives off a small branch to and receives a small branch from the C5 root. This interconnection is usually scarred and in most cases, a significant length of the phrenic nerve is enveloped in scar from upper trunk neuroma (Fig. 38‑5). Exposure between sternocleidomastoid and anterior scalene allows visualization of the distal course of phrenic nerve so that the phrenic nerve can be lysed from the neuroma. A thin layer of scar is left on the phrenic nerve to facilitate its safe handling. The interconnection between the phrenic nerve and the C5 root can be sacrificed without morbidity. With the phrenic nerve mobilized, the C5 and C6 nerve roots can be exposed. Partial release of the anterior scalene provides proximal exposure up to the bony intervertebral foramina. Dissection here should progress carefully given the rich venous plexus of the vertebral vessels just proximal. Care should also be taken to avoid traction on the phrenic nerve as it becomes particularly vulnerable with medial exposure.
Working from distal, the branches of the upper trunk can be identified under the clavicle where they are usually unscarred (Fig. 38‑6). The suprascapular nerve (SSN) parallels the omohyoid on its course toward the suprascapular notch and defines the lateral border of dissection. The anterior division of upper trunk (AD-UT) is the most medial branch and defines the medial border of dissection. The posterior division of upper trunk (PD-UT) is found in the middle. The three branches can be followed to their convergence at the trifurcation of the upper trunk, which is usually found at the level of the clavicle.
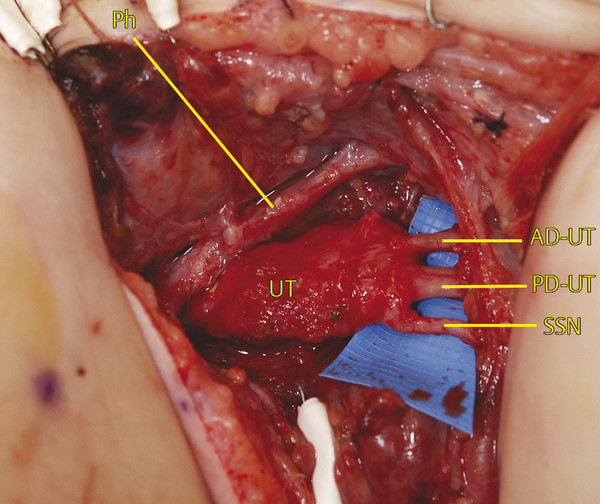
The posterior surface of neuroma can be difficult to dissect free from the middle scalene muscle due to scarification. As the dissection progresses proximally, the planes become clearer; however, the C5, C6, and C7 contributions to the long thoracic nerve become vulnerable as these branches dive through the middle scalene muscle proximally (Fig. 38‑7). Preservation of these branches can often be achieved to avoid scapular instability.

Retroclavicular Region
Due to the curvature of the spine, each sequential lower root is found inferior and dorsal to the root above. From the surgeon’s perspective, each sequential lower root will seem to be deeper in the exposure. The middle trunk is identified behind the upper trunk (Fig. 38‑8).

Further deep and slightly inferior dissection reveals the lower trunk. Proximal dissection to and between the anterior and middle scalenes reveals the respective proximal nerve roots. The C8 and T1 roots can be traced proximally and can be seen originating above and below the first cervical rib, respectively (Fig. 38‑9). Dissection too far inferior and anterior will quickly lead to the subclavian artery. This structure can be easily mistaken for the lower trunk, but it is pulsatile and can be confirmed with Doppler if needed. The subclavian artery should be carefully lysed from the brachial plexus. The trunks of the brachial plexus normally divide into divisions at or beneath the clavicle. The divisions are rarely scarred or injured.

Although the vertebral artery is generally not visualized, it traverses ventral to the C7 root to enter the foramen transversarium of the C6 vertebra. It can potentially be injured when releasing anterior scalene muscle while exposing proximal nerve roots.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








