Regulation of the Production and Activation of Eosinophils: Introduction
|
Eosinophils
Eosinophils develop in the bone marrow from multipotential, stem cell-derived CD34+ myeloid progenitor cells in response to eosinophilopoietic cytokines and growth factors (see Fig. 31-1). They are released into the circulation as mature cells.1–3 Important stimulatory cytokines and growth factors for eosinophils include interleukin (IL)-3, granulocyte macrophage colony stimulating factor (GM-CSF), and IL-5. Activated T cells likely are the principal sources of IL-3, GM-CSF, and IL-5 that induce eosinophil differentiation in bone marrow. However, depending on pathogenic stimuli, eosinophilopoietic cytokines may be released by other cell types, including mast cells, macrophages, natural killer cells, endothelial cells, epithelial cells, fibroblasts, and even eosinophils, themselves.4 IL-3 and GM-CSF are pluripotent cytokines that have effects on other hematopoietic lineages. IL-5 is the most selective eosinophil-active cytokine, but it is relatively late acting. Although it is both necessary and sufficient for eosinophil differentiation, IL-5 demonstrates maximum activity on the IL-5 receptor (IL-5R)-positive eosinophil progenitor pool that first is expanded by earlier acting pluripotent cytokines such as IL-3 and GM-CSF4; expression of the high affinity IL-5R is a prerequisite for eosinophil development. Exodus from the bone marrow also is regulated by IL-5. IL-3, GM-CSF, along with IL-5, promote survival, activation, and chemotaxis of eosinophils through binding to receptors that have a common β chain (CD131) with IL-5R, and unique α chains. See ch31etb0.1 for designations of many factors involved in eosinophil biology.
Figure 31-1
Eosinophils from undifferentiated hematopoietic cells to their fate in tissue. The images depict the eosinophil’s life from differentiation in the bone marrow to vascular transmigration to their fate in tissue with key factors noted.
* EXB4 likely has activities comparable with those of LTB4.
Preferred Designation | Other Designation or Abbreviation | Full Name |
|---|---|---|
AP-1 | Transcription factor activator protein-1 | |
Bcl-2 | B-cell leukemia/lymphoma-2 | |
BPI | Bactericidal/permeability-increasing protein | |
CD | Cluster of differentiation | |
CD11a | ITGAL | Integrin α L chain |
CD11b | αM integrin | |
CD11c | αX integrin | |
CD11a/CD18 | LFA-1 or αLβ2 | Leukocyte function-associated antigen-1 |
CD11b/CD18 | Mac-1 or CR3 | Complement receptor 3, receptor for C1q that also binds C4b, C3b, and iC3b |
CD15s | Sialyl-Lewis X | |
CD16 | FcγRIII | Fc γ receptor 3 |
CD18 | β2 integrin | |
CD25 | IL-2R | Interleukin 2 receptor |
CD30 | Ki-1, Ki-2, R4-4, Ber H2 or TNFRSF8 | TNF receptor superfamily member 8 |
CD32 | FcγRII | Fc γ receptor 2 |
CD34 | Marker on human stem cells that differentiate into blood cells | |
CD35 | CR3 | Complement receptor 3 |
CD44 | Hyaluronic acid receptor | |
CD45 | Common leukocyte antigen | |
CD48 | BCM1, SLAMF2 | B-cell activation marker 1, signaling lymphocyte activation molecule family member 2 |
CD49d/CD29 | VLA-4 | Very late antigen-4 |
CD50 | ICAM-3 | Intercellular adhesion molecule-3 |
CD52 | Campath | |
CD54 | ICAM-1 | Intercellular adhesion molecule-1 |
CD62L | l-selectin | |
CD64 | FcγRI | Fc γ receptor 1 |
CD69 | AIM | Activation inducer molecule |
CD89 | FcαRI | Fc α receptor 1 |
CD95 | Fas receptor | |
CD106 | VCAM-1 | Vascular cellular adhesion molecule-1 |
CD116 | GM-CSF | Granulocyte macrophage-colony stimulating factor receptor |
CD117 | SCF | Stem cell factor or c-kit receptor |
CD119 | IFN-γR | Interferon γ receptor |
CD120 | TNF-αR | Tumor necrosis factor α receptor |
CD123 | IL-3R | Interleukin-3 receptor |
CD124 | IL-4R | Interleukin-4 receptor |
CD125 | IL-5R | Interleukin-5 receptor |
CD129 | IL-9R | Interleukin-9 receptor and CD |
CD131 | Common β chain of IL-3, IL-5, GM-CSF receptors | |
CD132 | Common γ chain of IL-2, IL-4, IL-7, IL-9, IL-15 receptors | |
CD162 | PSGL-1 | p-selectin glycoprotein ligand-1 |
CD294 | CRTH2 | Chemoattractant receptor-homologous molecule expressed on Th2 lymphocytes |
CCL3 | MIP-1α | Macrophage inflammatory protein-1 α |
CCL5 | RANTES | Regulated on activation, normal T cells expressed and secreted |
CCL7 | MCP-3 | Monocyte chemotactic protein-3 |
CCL8 | MCP-2 | Monocyte chemotactic protein-2 |
CCL13 | MCP-4 | Monocyte chemotactic protein-4 |
CCL11 | Eotaxin-1 | |
CCL24 | Eotaxin-2 | |
CCL26 | Eotaxin-3 | |
CCR3 | CC chemokine receptor 3 | |
CLC | Charcot–Leyden crystal | |
CR | Complement receptor | |
CXCL8 | IL-8 | Interleukin-8 |
CXCL13 | BCA-1, BLC | B lymphocyte chemoattractant factor |
ECP or RNase 3 | RNase 3 or ECP | Eosinophil cationic protein |
EDN or RNase 2 | RNase 2 or EDN | Eosinophil-derived neurotoxin |
EPO | Eosinophil peroxidase | |
FIP1L1-PDGFRA | Fip1-like 1/platelet-derived growth factor receptor-α fusion gene | |
GM-CSF | Granulocyte macrophage-colony stimulating factor | |
HLA-DR | Human leukocyte antigens-DR | |
ICAM | Intercellular adhesion molecule | |
IL | Interleukin | |
IL-R | Interleukin receptor | |
IFN | Interferon | |
LT | Leukotriene | |
MBP | Major basic protein | |
MHC-II | Major histocompatibility complex, class II | |
MMP-9 | Matrix metalloproteinase-9 | |
mTOR | Mammalian target of rapamycin | |
NADPH oxidase | Nicotinamide adenine dinucleotide phosphate-oxidase | |
PAF | Platelet activating factor | |
PAR-2 | Protease-activated receptor-2 | |
PDGF | Platelet-derived growth factor | |
PG | Prostaglandin | |
PIR-B | Paired immunoglobulin-like receptor B | |
SNAP | Soluble N-ethylmaleimide-sensitive factor attachment protein | |
SNARE | SNAP receptor | |
TGF | Transforming growth factor | |
TLR | Toll-like receptor | |
TNF | Tumor necrosis factor | |
TSLP | Thymic stromal lymphopoietin (TSLP) | |
VAMP | Vesicle-associated membrane protein |
![]() Eosinophils and basophils share the CD34+ progenitor, also referred to as the “eosinophil/basophil colony forming unit.”5 This hybrid cell is characterized by granule contents that have features of both cell types and by the expression of common receptors, the high-affinity IL-5R and the CC chemokine receptor 3 (CCR3), which binds most eosinophil-specific chemokines.6 This hybrid cell has been identified in the circulation of patients with atopic dermatitis and in inflamed tissues of patients with allergic diseases.7 Cells within these tissues produce eosinophilopoietins; therefore, extramedullary eosinophilopoiesis may occur at sites of allergic inflammation.8 Eosinophil/basophil progenitors also are altered in cord blood of infants at risk of atopy and asthma, suggesting that hematopoietic processes underlying the allergic phenotype may begin in the prenatal period.8
Eosinophils and basophils share the CD34+ progenitor, also referred to as the “eosinophil/basophil colony forming unit.”5 This hybrid cell is characterized by granule contents that have features of both cell types and by the expression of common receptors, the high-affinity IL-5R and the CC chemokine receptor 3 (CCR3), which binds most eosinophil-specific chemokines.6 This hybrid cell has been identified in the circulation of patients with atopic dermatitis and in inflamed tissues of patients with allergic diseases.7 Cells within these tissues produce eosinophilopoietins; therefore, extramedullary eosinophilopoiesis may occur at sites of allergic inflammation.8 Eosinophil/basophil progenitors also are altered in cord blood of infants at risk of atopy and asthma, suggesting that hematopoietic processes underlying the allergic phenotype may begin in the prenatal period.8
![]() Studies in mice corroborate the primary importance of the cytokine, IL-5, and the CC chemokine, CCL11 (eotaxin-1).9 For example, IL-5 transgenic mice develop peripheral blood and tissue eosinophilia.10,11 Furthermore, administration of neutralizing IL-5 antibodies in wild-type mice leads to a significant reduction in baseline levels of circulating eosinophils and diminished tissue eosinophilia in response to infection with various parasites or allergen challenge. It is important to note that despite the massive eosinophilia observed in IL-5 transgenic mice, these mice do not develop the tissue damage observed in eosinophilic diseases. This is likely because eosinophils also must be activated to degranulate and/or release their inflammatory mediators. Mice genetically deficient in IL-5 have little or no blood eosinophilia, but have eosinophils in their bone marrow, albeit at reduced levels. A mouse strain deficient in the common β chain, CD131, shared by the IL-3, GM-CSF, and IL-5 receptors have reduced lung eosinophilia in asthma models,12 whereas mice deficient in both CCL11 and IL-5 have even greater reduction in tissue eosinophils in asthma models.13 The highly eosinophil-specific expression of eosinophil peroxidase (EPO) has been exploited for the development of an eosinophil-deficient mouse (so-called PHIL mice, “eosinoPHIL” minus “eosino” mice) in which expression of a toxin is molecularly linked to EPO expression, resulting in eosinophil death before they leave the bone marrow.14 These eosinophil-less mice have subsequently been employed in various disease models, including asthma. Importantly, the mouse models with reduced (IL-5 knockout) or no eosinophils (PHIL) have normal life spans, reproduce normally, do not have increased infections, increased malignancies, or other apparent health defects (personal communications with Paul S. Foster, Ph.D. University of Newcastle, Newcastle, Australia, and James J. Lee, Ph.D., Mayo Clinic, Scottsdale, Arizona). A human correlate to the IL-5 transgenic mouse is the recently recognized lymphocytic variant of hypereosinophilic syndromes (see Chapter 36), in which a clonal population of T cells producing IL-5 causes persistent and profound eosinophilia and which often is responsive to anti-IL-5 (mepolizumab) therapy.15–17 The ability to propagate eosinophils from bone marrow and umbilical cord stem cells is dependent on IL-5 and further substantiates its role as the most critical cytokine for eosinophil proliferation, maturation, terminal differentiation, and survival.18,19 In summary, allergen challenge, inflammatory eosinophilic disorders, and parasitic infections induce the production and release of eosinophil precursors from the bone marrow in response to endocrinological actions of cell-derived IL-5, with a contribution by CCL11.
Studies in mice corroborate the primary importance of the cytokine, IL-5, and the CC chemokine, CCL11 (eotaxin-1).9 For example, IL-5 transgenic mice develop peripheral blood and tissue eosinophilia.10,11 Furthermore, administration of neutralizing IL-5 antibodies in wild-type mice leads to a significant reduction in baseline levels of circulating eosinophils and diminished tissue eosinophilia in response to infection with various parasites or allergen challenge. It is important to note that despite the massive eosinophilia observed in IL-5 transgenic mice, these mice do not develop the tissue damage observed in eosinophilic diseases. This is likely because eosinophils also must be activated to degranulate and/or release their inflammatory mediators. Mice genetically deficient in IL-5 have little or no blood eosinophilia, but have eosinophils in their bone marrow, albeit at reduced levels. A mouse strain deficient in the common β chain, CD131, shared by the IL-3, GM-CSF, and IL-5 receptors have reduced lung eosinophilia in asthma models,12 whereas mice deficient in both CCL11 and IL-5 have even greater reduction in tissue eosinophils in asthma models.13 The highly eosinophil-specific expression of eosinophil peroxidase (EPO) has been exploited for the development of an eosinophil-deficient mouse (so-called PHIL mice, “eosinoPHIL” minus “eosino” mice) in which expression of a toxin is molecularly linked to EPO expression, resulting in eosinophil death before they leave the bone marrow.14 These eosinophil-less mice have subsequently been employed in various disease models, including asthma. Importantly, the mouse models with reduced (IL-5 knockout) or no eosinophils (PHIL) have normal life spans, reproduce normally, do not have increased infections, increased malignancies, or other apparent health defects (personal communications with Paul S. Foster, Ph.D. University of Newcastle, Newcastle, Australia, and James J. Lee, Ph.D., Mayo Clinic, Scottsdale, Arizona). A human correlate to the IL-5 transgenic mouse is the recently recognized lymphocytic variant of hypereosinophilic syndromes (see Chapter 36), in which a clonal population of T cells producing IL-5 causes persistent and profound eosinophilia and which often is responsive to anti-IL-5 (mepolizumab) therapy.15–17 The ability to propagate eosinophils from bone marrow and umbilical cord stem cells is dependent on IL-5 and further substantiates its role as the most critical cytokine for eosinophil proliferation, maturation, terminal differentiation, and survival.18,19 In summary, allergen challenge, inflammatory eosinophilic disorders, and parasitic infections induce the production and release of eosinophil precursors from the bone marrow in response to endocrinological actions of cell-derived IL-5, with a contribution by CCL11.
The interactions of eosinophilopoietic factors with their receptors stimulate a cascade of complex biochemical events through signal transduction. Signaling events progress in four steps: (1) juxtamembranous signaling in which membrane-anchored tyrosine kinases and lipid kinases are activated; (2) signal interfacing which serves to transduce juxtamembranous signals to cytosolic signals; (3) mobile signaling in which cytosolic signaling molecules translocate from the receptor site to other cellular compartments including the nucleus, mitochondria, and cytoskeleton; and (4) transcription activation resulting from nuclear translocation and initiation of gene transcription. Studies have shown the pivotal role of IL-5 in immune responses involving eosinophils through receptor-driven signaling.20 IL-5 binds to the α chain of the IL-5R and induces recruitment of the common β (βc) chain to IL-5R. Janus kinase (JAK)2 tyrosine kinase is constitutively associated with IL-5Rα, and JAK1 tyrosine kinase with IL-5Rβc; both are activated with IL-5 binding as part of the juxtamembranous step. Lyn and Fes are other tyrosine kinases involved in the first step; these tyrosine kinases also are activated by IL-3 and GM-CSF. Adaptor proteins, src homologues and collagen (Shc), SH2-containing phosphatase-2 (SHP-2), growth factor receptor-bound protein 2 (Grb2), Vav, and lipid kinases, phosphatidylinositol 3-kinase (PI-3K), function in the interfacing step. The activation of JAK2 and signal transducer and activator of transcription (STAT) 5 is essential for IL-5 dependent signal transduction. The Ras GTPase-extracellular signal-regulated kinase (Ras-ERK) and also known as Ras-mitogen-activated protein kinase (Ras-MAPK) pathway, in addition to the JAK2-STAT5 pathway, is important in IL-5 signaling in the mobile step. The JAK-STAT and Ras-MAPK pathways converge at various levels in IL-5 signaling of eosinophils. IL-5 induces the expression of cytokine-inducible SH2 protein (CIS) and JAK-binding protein (JAB), which is one of the negative feedback loops in the regulation of IL-5 signaling. Multiple other interactive signal transduction pathways induce and regulate gene expression for eosinophil growth, development, activation, and survival.21,22 Much of the discovery in these pathways has been in murine systems with presumed general applicability to humans. However, at least part of the immune response to IL-5 in mice is NOT part of the biological effect in humans, i.e., in mice, IL-5 enhances several functions of B cells, including immunoglobulin production, growth, and differentiation, whereas human B-cells are influenced by IL-5 only in the presence of specific cytokines and under certain conditions.20 However, human IL-5 does act on T cells by increasing the expression of IL2Rα and augmenting cytotoxic T cell generation.23
![]() Eosinophil development occurs as a result of functional interactions among various transcription factors.24 The key transcription factors involved in eosinophil lineage commitment and terminal differentiation are GATA-1, FOG-1 (friend of GATA-1), C/EBPα (CCAAT enhancer-binding protein α), and the ets (E-twenty six) family transcription factor, PU.1.4 GATA-1 functions primarily to facilitate the differentiation of granulocyte–macrophage progenitors into eosinophils. As a result, GATA-1-deficient mice do not develop eosinophils, and deletion of a specific GATA binding site of the mouse GATA-1 promoter (ΔdblGATA mice) results in strains of mice in which terminal differentiation of eosinophils is prevented.25,26 FOG-1 functions to antagonize GATA-1 activity and must be downregulated for eosinophil development to occur.19,27 Mice deficient in C/EBPα. are devoid of all granulocytes,28 and mice congenitally deficient in PU.1 are unable to generate terminally differentiated eosinophils.4 As may be predicted, many of these transcription factors are important in generating eosinophil lineage-specific granule proteins, such as major basic protein (MBP)-1, along with CCR3 and the α subunit of IL-5R.29 For progenitor cells to become committed to eosinophil development requires concomitant expression of C/EBPα, PU.1, and low-to-moderate GATA-1, with no expression of FOG-1. Another member of the C/EBP transcription factor family, C/EBPϵ, is required for terminal differentiation and functional maturation of eosinophils and neutrophils. C/EBPϵ-null (knockout) mice lack functionally mature granulocytes.
Eosinophil development occurs as a result of functional interactions among various transcription factors.24 The key transcription factors involved in eosinophil lineage commitment and terminal differentiation are GATA-1, FOG-1 (friend of GATA-1), C/EBPα (CCAAT enhancer-binding protein α), and the ets (E-twenty six) family transcription factor, PU.1.4 GATA-1 functions primarily to facilitate the differentiation of granulocyte–macrophage progenitors into eosinophils. As a result, GATA-1-deficient mice do not develop eosinophils, and deletion of a specific GATA binding site of the mouse GATA-1 promoter (ΔdblGATA mice) results in strains of mice in which terminal differentiation of eosinophils is prevented.25,26 FOG-1 functions to antagonize GATA-1 activity and must be downregulated for eosinophil development to occur.19,27 Mice deficient in C/EBPα. are devoid of all granulocytes,28 and mice congenitally deficient in PU.1 are unable to generate terminally differentiated eosinophils.4 As may be predicted, many of these transcription factors are important in generating eosinophil lineage-specific granule proteins, such as major basic protein (MBP)-1, along with CCR3 and the α subunit of IL-5R.29 For progenitor cells to become committed to eosinophil development requires concomitant expression of C/EBPα, PU.1, and low-to-moderate GATA-1, with no expression of FOG-1. Another member of the C/EBP transcription factor family, C/EBPϵ, is required for terminal differentiation and functional maturation of eosinophils and neutrophils. C/EBPϵ-null (knockout) mice lack functionally mature granulocytes.
![]() In humans, a novel loss-of-function mutation in the C/EBPϵ transcription factor results in failure of terminal differentiation of both eosinophils and neutrophils along with failed expression of secondary/specific granule protein genes in both granulocytes.30 These patients are severely immunocompromised and develop frequent bacterial infections. As knowledge of the intricate interactions among transcription factors that direct eosinophil commitment and differentiation continues to unfold, new understanding of eosinophil regulation will emerge, including potential therapies for eosinophil-associated diseases.
In humans, a novel loss-of-function mutation in the C/EBPϵ transcription factor results in failure of terminal differentiation of both eosinophils and neutrophils along with failed expression of secondary/specific granule protein genes in both granulocytes.30 These patients are severely immunocompromised and develop frequent bacterial infections. As knowledge of the intricate interactions among transcription factors that direct eosinophil commitment and differentiation continues to unfold, new understanding of eosinophil regulation will emerge, including potential therapies for eosinophil-associated diseases.
(See Fig. 31-2)
Figure 31-2
Products of eosinophils and localization of distinctive granule proteins. The eosinophil produces myriad products, including toxic granule proteins, which implicate its role in disease pathogenesis. The characteristic eosinophil granules are coarse and, as their name implies, eosinophilic upon staining with eosin. Distinctive granule proteins are localized to core and matrix portions of the specific cytoplasmic granules. A. An intact dermal eosinophil with its distinctive granules and typical bilobed nucleus. B. Characteristic specific (secondary) eosinophil granule with electron dense crystalline core and radiolucent matrix showing localization of distinctive granule proteins.
Mature eosinophils are 12–17 μm in diameter and, therefore, slightly larger than neutrophils. They typically have a bilobed nucleus with highly condensed peripheral chromatin. Eosinophils have distinctive cytoplasmic granules, demonstrated by their staining properties with acidic dyes such as eosin and by their unique electron microscopic appearance. These specific or secondary granules are composed of an electron-dense core and a less electron dense matrix, the core being a crystalline lattice by electron microscopy. In cross section, the eosinophil contains approximately 30 of these membrane-bound, core-containing, secondary granules.1 Five highly basic proteins are found within the granules: (1) major basic protein (MBP)-1, (2) MBP-2, (3) eosinophil-derived neurotoxin (EDN) also known as ribonuclease (RNase)2, (4) eosinophil cationic protein (ECP) also known as RNase3, and (5) eosinophil peroxidase (EPO). Several other types of proteins are found in secondary granules and include enzymes, cytokines, growth factors, and chemokines. Eosinophils contain three other types of cytoplasmic granules, referred to as (1) primary granules, (2) small granules, and (3) secretory vesicles. Primary granules are of variable size, round, uniformly dense, present in 1–3 per electron microscopic cross section, and more common in immature eosinophilic promyelocytes. These granules may contain Charcot–Leyden crystal protein (also known as galectin-10), which can be found in neutrophils as well31; Charcot–Leyden crystals (CLCs) are characteristically found in asthmatic sputum and in feces from patients with helminth infections or eosinophilic gastroenteritis. Small granules contain acid phosphatase and arylsulfatase and are present at 2–8 per electron microscopic cross section. Secretory vesicles, also referred to as tubulovesicular structures or microgranules, are characterized by their small, dumbbell-shaped appearance and their albumin content. They are the most abundant granules in number, with approximately 160 per electron microscopic cross section. Normal eosinophils contain varying numbers of nonmembrane-bound lipid bodies, which are the principal stores of arachidonic acid. Lipid bodies also contain the enzymes, cyclooxygenase, 5- and 15-lipoxygenase, which are required to synthesize prostaglandins, leukotrienes (LTs), and eoxins (vide infra), and are increased in activated eosinophils.1
![]() Eosinophils likely evolved from an ancestral phagocytic cell that existed in primitive invertebrates. When immune functions emerged separately from digestive functions, cells resembling granulocytes, including eosinophilic cells, evolved. Eosinophils have been described in numerous species of fish and are also present in frogs. They are present in reptiles although they may not contain a crystalloid internus.32 Reptiles are pivotal on the evolutionary scale because they are progenitors of both avian and mammalian classes. Eosinophils are found in numerous mammals other than humans. Controversy exists as to the evolutionary pressures that directed the development of these cells and their current biological functions. Eosinophils are likely an important part of the innate and adaptive immune response. The effector response of eosinophils may also contribute to the physiological and pathological reactions associated with disease.33 Through elaboration of remodeling and fibrogenic growth factors, eosinophils may have important roles in the maintenance of tissue homeostasis, wound healing, and repair.34,35 Eosinophils regulate mast cell functions through release of granule proteins and cytokines, and, in a reciprocal manner, mast cells also activate eosinophils.36,37
Eosinophils likely evolved from an ancestral phagocytic cell that existed in primitive invertebrates. When immune functions emerged separately from digestive functions, cells resembling granulocytes, including eosinophilic cells, evolved. Eosinophils have been described in numerous species of fish and are also present in frogs. They are present in reptiles although they may not contain a crystalloid internus.32 Reptiles are pivotal on the evolutionary scale because they are progenitors of both avian and mammalian classes. Eosinophils are found in numerous mammals other than humans. Controversy exists as to the evolutionary pressures that directed the development of these cells and their current biological functions. Eosinophils are likely an important part of the innate and adaptive immune response. The effector response of eosinophils may also contribute to the physiological and pathological reactions associated with disease.33 Through elaboration of remodeling and fibrogenic growth factors, eosinophils may have important roles in the maintenance of tissue homeostasis, wound healing, and repair.34,35 Eosinophils regulate mast cell functions through release of granule proteins and cytokines, and, in a reciprocal manner, mast cells also activate eosinophils.36,37
In mammals, such as the mouse and humans, the eosinophil is released as a mature cell into the circulation from the bone marrow, but is present in the blood only transiently, ranging from 8–18 hours. Eosinophils comprise a small portion, normally 6% or less, of circulating leukocytes. They are primarily tissue dwelling cells, but only in certain tissues in humans, with an average tissue life span of 2–5 days. This may be prolonged by cytokines that increase eosinophil survival for up to 14 days. Under normal circumstances, a balance exists between bone marrow production and release of eosinophils, their time in circulation, and their entrance into tissues. Changes in any one of the compartments causes an increase or decrease in circulating and tissue eosinophils. Eosinophilia in blood or tissue or both is associated with helminthiasis, allergic hypersensitivity, and other pathological conditions. In humans, bone marrow, spleen, lymph node, thymus, and gastrointestinal tract from the stomach through the colon, sparing the esophagus, are the only tissues in which eosinophils normally reside.38 Furthermore, the gastrointestinal tract is the only organ other than bone marrow in which extracellular eosinophil granule protein deposition is observed even under homeostatic conditions. Eosinophils and their granule proteins are found in the lamina propria in normal gastrointestinal tract and are not found in Peyer’s patches or epithelium. Eosinophils may be important for thymocyte deletion based on the localization of eosinophils within the thymus and the timing of their migration during the neonatal period.39 Murine observations indicate that eosinophils are also important for postnatal mammary gland and uterine development, and their recruitment into the uterus heralds estrus. Although eosinophils, themselves, are not known to participate in human reproduction, eosinophil proMBP-1 is expressed in the uterus by placental X and giant cells during pregnancy, and its production peaks 2–3 weeks before parturition.40,41 The recruitment of eosinophils to the gastrointestinal, thymic, uterine, and mammary tissues is under the control of the CC chemokine, CCL11.42,43
Once eosinophils enter tissues, most do not recirculate. Several possible mechanisms exist for removal of tissue eosinophils. These include shedding of the cells across mucosal surfaces into the lumen of the intestinal or respiratory tract, engulfment of apoptotic eosinophils by macrophages, and lysis or degranulation with cellular degeneration. In various inflammatory conditions, including those affecting the skin (Chapter 36), striking numbers of free granules and/or eosinophil granule protein deposition are present in the absence of intact eosinophils.1 Studies recently have revealed that isolated eosinophil granules express extracellular domains for interferon (IFN)-γ receptor and CCR3 and, upon stimulation, respond independently as organelles by releasing ECP.44
Shortly after their discovery by Paul Ehrlich in 1879, eosinophils were observed in association with helminth infections. Theories have been promulgated that eosinophils are important for host defense against parasites spawning numerous studies.45 For example, in vitro studies demonstrated that eosinophils are cytotoxic to large nonphagocytosable organisms, such as multicellular helminthic parasites. Eosinophils bind to host-derived immunoglobulins and complement components on the surface of their targets (so-called antibody- or complement-) dependent cytotoxicity. They also bind to carbohydrate ligands expressed on parasites, such as the Lewisx-related molecules, and cell adhesion molecules similar to selectins. Eosinophils are activated to release their granule products with deposition of these biologically active proteins in and around the parasites causing disruption of the parasite’s integument and, ultimately, death of the organism. The granule proteins have different effects. ECP produces fragmentation and disruption whereas MBP-1 produces a distinctive ballooning detachment of the tegumental membrane, and EDN is active only at high concentrations causing crinkling of the tegumental membrane.46 However, in murine models in which blood, marrow, and tissue eosinophilia is largely abolished by neutralizing IL-5 activity, the intensities of primary or secondary parasitic infection are unchanged indicating that eosinophils have little or no role in parasitic host defense in these models.1 The results must be interpreted cautiously because mouse and human eosinophils have functional differences, and mice are not natural hosts of many of the parasites tested experimentally.
Eosinophils also release cytotoxic granule proteins onto the surface of fungal organisms and into the extracellular milieu in fungal infections. Eosinophils kill fungi in a contact-dependent manner. Eosinophils adhere to the fungal cell wall component, β-glucan, via a β2-integrin surface molecule, CD11b.47 Eosinophils do not express other common fungal receptors, such as dectin-1 and lactosylceramide, and, specifically, do not react with chitin. However, chitin, which is a polymer that confers structural rigidity to fungi, helminths, crustaceans, and insects, induces accumulation of eosinophils in tissues through production of LT B4 in mice.48 Eosinophils also are activated by fungal organisms that release proteases, such as Alternaria, through protease-activated receptors (PARs). For example, fungal aspartate protease activates eosinophils through PAR-2 and, thereby, mediates eosinophils’ innate responses to certain fungi.49
As a granulocyte, the eosinophil is capable of phagocytosing and killing bacteria and other small microbes in vitro, but eosinophils cannot effectively defend against bacterial infections when neutrophil function is deficient. Nevertheless, recent investigations reveal that eosinophils may have a role in innate immunity against bacteria using a unique mechanism, DNA trap.50 Eosinophils rapidly release mitochondrial DNA when exposed to bacteria, a complement component, C5a, or CCR3 ligands. The traps contain eosinophil granule proteins, ECP and MBP, and have antimicrobial effects. In the extracellular space, the granule proteins and mitochondrial DNA form structures that bind and kill bacteria both in vitro and in vitro. Eosinophils, unlike neutrophils, do not undergo cell death as part of this process. This may be an important innate immune response, particularly in mucosal epithelium.50
Another protective function that eosinophils may have is in viral infections. Eosinophils and their granule proteins are increased in the respiratory tracts of patients with respiratory syncytial virus, an RNA-viral infection. EDN (RNase2) and ECP (RNase3), eosinophil granule matrix proteins, are ribonucleases (vide infra). Purified eosinophils, as well as EDN and ECP individually, reduced viral titers when added to respiratory syncytial viral suspensions. In mice, at least 11 eosinophil-associated ribonucleases degrade single stranded RNA containing viruses.51 Despite divergence of the coding regions, conserved eosinophil ribonuclease activity across species suggests a strong evolutionary pressure to preserve this critical enzymatic activity.51 In other studies, pretreatment of parainfluenza-infected guinea pigs with anti-IL-5, to reduce numbers of eosinophils, strikingly increased viral load in the airways. Viruses, including parainfluenza virus, respiratory syncytial virus, and rhinovirus, induce the release of another eosinophil granule protein, EPO, when coincubated with antigen-presenting cells and T cells.52 Paradoxically, eosinophils may be a potential reservoir for the human immunodeficiency virus (HIV)-1.53
Eosinophils may have other roles in immune responses as well. Through MHC class II expression and IL-1α production, they can function as antigen-presenting cells for a variety of viral, parasitic, and microbial antigens, including staphylococcal superantigens, and allergens.54,55 Eosinophils are recruited to secondary lymphoid structures to promote the proliferation of effector T cells although they are unable to affect naïve T cells.56 Eosinophils, as sources of cytokines, influence T cell-dependent responses.1 In keeping with the prominence of eosinophils in allergic disorders, eosinophils are involved in T cell polarization favoring Th2 by promoting Th1 apoptosis in addition to their influence via cytokine expression.54,57–60
The activities of eosinophil-derived products include direct cytotoxic effects on structural cells and microbes, increased vascular permeability, procoagulant effects, innate immune responses to some parasites, viruses, fungi and tumor cells, enhancement of leukocyte migration, amplification of effector T-cell responses and, possibly even mammary gland development. Collectively, these varied biologic actions provide the pathophysiological basis for the signs and symptoms observed in eosinophil-associated diseases.
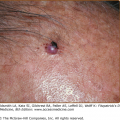
Eosinophils in lymph nodes and spleen are especially increased after allergen exposures or microbial insults.61,62 Eosinophils have been found in several cancers, particularly in lymphomas, leukemias, and colon cancer. Clinical studies indicate that certain tumors associated with tissue and/or peripheral eosinophilia have a more favorable prognosis,63 whereas in other tumors, they are thought to confer a poor prognosis, such as nodular sclerosing Hodgkin disease, Sézary syndrome, and gastric carcinomas. In Sézary syndrome (see Chapter 145), the tumor cells produce IL-5 and, therefore, are responsible for the eosinophilia, which is a reflection of tumor burden.64 Where eosinophilia is a good prognostic factor, eosinophils are considered to be part of an effective host response to the tumor.65,66
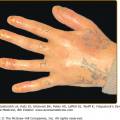
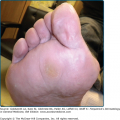
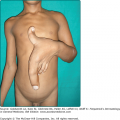
![]() The specific nature of eosinophil-dependent antitumor activity has not been sufficiently explored but may include, in addition to direct cytotoxic effects on tumor cells, tumor antigen presentation, vascular compromise via microthrombi formation, or synergism with the antitumor response of other leukocytes. There are many gastrointestinal tract disorders in which eosinophil numbers are substantially increased such as drug reactions, helminthic infections, inflammatory bowel disease, gastroesophageal reflux, and the primary eosinophilic gastrointestinal disorders (EGID) including eosinophilic esophagitis, eosinophilic gastritis, and eosinophilic gastroenteritis. The skin and respiratory tract, in contrast to lymphatic and gastrointestinal tissues, normally are devoid of eosinophils. Both intact and degranulated eosinophils are a significant component of the inflammatory infiltrate in many cutaneous diseases as described in Chapter 36. Eosinophils were identified in anaphylaxis and immunoglobulin (Ig) E-mediated hypersensitivity where they were originally hypothesized to play a protective role. They were subsequently defined as a component of Th2 immunity. Asthma is the prototypic disease in which eosinophil involvement has been most extensively characterized.67–69
The specific nature of eosinophil-dependent antitumor activity has not been sufficiently explored but may include, in addition to direct cytotoxic effects on tumor cells, tumor antigen presentation, vascular compromise via microthrombi formation, or synergism with the antitumor response of other leukocytes. There are many gastrointestinal tract disorders in which eosinophil numbers are substantially increased such as drug reactions, helminthic infections, inflammatory bowel disease, gastroesophageal reflux, and the primary eosinophilic gastrointestinal disorders (EGID) including eosinophilic esophagitis, eosinophilic gastritis, and eosinophilic gastroenteritis. The skin and respiratory tract, in contrast to lymphatic and gastrointestinal tissues, normally are devoid of eosinophils. Both intact and degranulated eosinophils are a significant component of the inflammatory infiltrate in many cutaneous diseases as described in Chapter 36. Eosinophils were identified in anaphylaxis and immunoglobulin (Ig) E-mediated hypersensitivity where they were originally hypothesized to play a protective role. They were subsequently defined as a component of Th2 immunity. Asthma is the prototypic disease in which eosinophil involvement has been most extensively characterized.67–69
![]() Along with parasitic infections, asthma was recognized soon after eosinophil discovery as a disease in which eosinophils were prominent. The findings of two disparate disorders, parasitism and asthma, linked by eosinophils prompted the more recent concept that the mechanisms by which eosinophils participate in host defense may have deleterious effects to the host. Investigations followed and revealed that eosinophils are unique among circulating leukocytes in their amazing abilities to wage chemical warfare. They are endowed with highly toxic granule proteins and an arsenal of enzymes that are released upon activation or with cell lysis that inflict damage on biological targets.
Along with parasitic infections, asthma was recognized soon after eosinophil discovery as a disease in which eosinophils were prominent. The findings of two disparate disorders, parasitism and asthma, linked by eosinophils prompted the more recent concept that the mechanisms by which eosinophils participate in host defense may have deleterious effects to the host. Investigations followed and revealed that eosinophils are unique among circulating leukocytes in their amazing abilities to wage chemical warfare. They are endowed with highly toxic granule proteins and an arsenal of enzymes that are released upon activation or with cell lysis that inflict damage on biological targets.
The eosinophil contains and produces a myriad of factors that implicate its role in inflammation and tissue destruction and remodeling (see Fig. 31-2).70 Products released by eosinophils include chemoattractants, colony-stimulating factors, and endothelial-activating cytokines. In addition to toxic cationic proteins from specific granules and oxidative products released into tissues following activation, these factors include arachidonic acid-derived lipids, hydrolytic enzymes, neuropeptides, colony-stimulating factors, and cytokines/chemokines that facilitate further leukocyte recruitment to sites of inflammation (see Fig. 31-2). Surface molecule expression is important in all aspects of eosinophil biology from promoting growth and differentiation to eosinophil trafficking into tissue to activation and/or priming of the cells to senescence. Numerous membrane factors are expressed on eosinophils that further direct eosinophil biological effects. (See ch31etb0.1 for designations of various eosinophil factors.)
Among the products of eosinophils that are most damaging to the host are the specific granule’s cationic proteins. Furthermore, the granule proteins are markers of eosinophil activity because the eosinophil often loses its characteristic morphology through cytolysis in tissues.71 Knowledge of their biological actions provides insight into their functions in human disease. Once deposited, the granule proteins persist in tissues for extended times—EPO for 1 week, ECP for 2 weeks, EDN for 2.5 weeks, and MBP-1 for 6 weeks.72 Each of these proteins have been shown to induce direct tissue damage to both host cells, including myocytes, endothelium, neurons, epithelium, and smooth muscle, and to microbes (vide supra). For example, MBP-1, ECP, or EPO application to airway epithelium in primates produces ciliostasis, desquamation, and hyperreactivity of respiratory smooth muscle mimicking the pathology of asthma.73,74 Damage to endothelium in eosinophilic endomyocardial disease is thought to be the initiating factor in the cardiomyopathy observed in the hypereosinophilic syndromes (see Chapter 36).75 All four of the cationic granule proteins [(1) EPO, (2) ECP, (3) EDN, and (4) MBP-1] likely contribute to the edema observed in skin diseases due to their vasodilatory effects with contribution from mast cells and basophil histamine release by MBP-1.76 Eosinophil granule proteins stimulate various cells in addition to mast cells and basophils, including neutrophils and platelets. Nodules, eosinophilia, rheumatism, dermatitis and swelling (NERDS), episodic angioedema with eosinophilia (Gleich syndrome), urticaria, eosinophilic cellulitis (Wells syndrome), and insect bite reactions demonstrate variable degrees of edema that are probably explained, at least in part, by this mechanism (see Chapter 36). Eosinophil granule proteins injected into skin produce lesions including dose-dependent wheal-and-flare reactions by MBP and ulcerations by ECP and EDN.77,78 Wound healing is delayed in the presence of eosinophils and eosinophil granule proteins.79,80 Eosinophils, through activities of granule proteins, have procoagulant activity. Thromboses have developed in the hypereosinophilic syndromes, including several case reports of hepatic vein obstruction (Budd–Chiari syndrome). The thromboses could be the result of direct endothelial damage or due to the ability of MBP and ECP to neutralize heparin. In addition, MBP is a strong platelet agonist, and platelet-activating factor (PAF), which is released by eosinophils, causes platelet aggregation.1
Major basic protein (MBP) comprises the crystalloid core of the specific eosinophil granule. It was so named because it accounts for a major portion, about 55% (in guinea pig), of the eosinophil granule protein, and has a high isoelectric point, calculated at greater than pH 11, so strongly basic that it cannot be measured accurately. It is now known that MBP is expressed as two homologs, MBP-1 and MBP-2, coded by different genes on chromosome 11.
![]() MBP-1 and, presumably, MBP-2 are translated as a preproprotein with a strongly acidic proportion. The combination of the prosection with MBP-1 or MBP-2 yields a molecule with a relatively neutral pI, 6.2. ProMBP-1, and possibly proMBP-2, lacks the cytotoxic properties of MBP-1 and MBP-2; synthesis as a neutral proprotein may function to protect the cell during transport from the Golgi apparatus to the granule. ProMBP-1 is the predominant form of MBP-1 in the blood of pregnant women and circulates as a complex with pregnancy-associated plasma protein-A (PAPP-A), with angiotensin, and with complement component, C3dg. It localizes to placental X cells and functions as a novel enzyme inhibitor of PAPP-A to modify its ability to release active insulin-like growth factor during primate pregnancy.1 Both MBP-1 and MBP-2 are present in eosinophil granules; MBP-1 also is present in basophils in a much lesser concentration than in eosinophils, but MBP-2 is not in basophils. Mature eosinophils lose the ability to transcribe mRNA encoding MBP-1 indicating that all MBP-1 stored in the crystalloid granule cores is synthesized during early eosinophil development. MBP-2 is approximately 100 times less basic than MBP-1, with calculated pI of 8.7. MBP-1 and MBP-2 have 42 identical amino acids of the approximately 117 in each of these proteins. The crystal structure of MBP-1 indicates that it is a member of the C-type lectin family but does not have all the biochemical activities of lectins. Rather, MBP-1 and MBP-2 have many other activities. Comparative analyses of the biological effects of MBP-1 and MBP-2 demonstrate that they are similar in cytotoxic and cytostimulatory effects, but with reduced potency of MBP-2. Most of the eosinophil granule protein activities have been characterized for MBP-1.
MBP-1 and, presumably, MBP-2 are translated as a preproprotein with a strongly acidic proportion. The combination of the prosection with MBP-1 or MBP-2 yields a molecule with a relatively neutral pI, 6.2. ProMBP-1, and possibly proMBP-2, lacks the cytotoxic properties of MBP-1 and MBP-2; synthesis as a neutral proprotein may function to protect the cell during transport from the Golgi apparatus to the granule. ProMBP-1 is the predominant form of MBP-1 in the blood of pregnant women and circulates as a complex with pregnancy-associated plasma protein-A (PAPP-A), with angiotensin, and with complement component, C3dg. It localizes to placental X cells and functions as a novel enzyme inhibitor of PAPP-A to modify its ability to release active insulin-like growth factor during primate pregnancy.1 Both MBP-1 and MBP-2 are present in eosinophil granules; MBP-1 also is present in basophils in a much lesser concentration than in eosinophils, but MBP-2 is not in basophils. Mature eosinophils lose the ability to transcribe mRNA encoding MBP-1 indicating that all MBP-1 stored in the crystalloid granule cores is synthesized during early eosinophil development. MBP-2 is approximately 100 times less basic than MBP-1, with calculated pI of 8.7. MBP-1 and MBP-2 have 42 identical amino acids of the approximately 117 in each of these proteins. The crystal structure of MBP-1 indicates that it is a member of the C-type lectin family but does not have all the biochemical activities of lectins. Rather, MBP-1 and MBP-2 have many other activities. Comparative analyses of the biological effects of MBP-1 and MBP-2 demonstrate that they are similar in cytotoxic and cytostimulatory effects, but with reduced potency of MBP-2. Most of the eosinophil granule protein activities have been characterized for MBP-1.
MBP-1 directly damages helminths and also lethally damages mammalian cells and tissues, examples of which are its ability to cause exfoliation of bronchial epithelial cells and to kill tumor cells. MBP-1 exerts its effects by increasing cell membrane permeability through surface charge interactions leading to disruption of the cell surface lipid bilayer.81 MBP-1 and MBP-2, but none of the other eosinophil granule proteins, stimulate histamine and LTC4 release from human basophils. Further, MBP-1 and MBP-2 stimulate neutrophils, inducing release of superoxide, lysozyme, and IL-8. MBP-1 and EPO are potent platelet agonists causing release of 5-hydroxytryptamine and promoting clotting.
Eosinophil peroxidase (EPO) is highly basic, pI 10.8, localized in the matrix of the specific eosinophil granule and is a key participant in generating reactive oxidants and free radical species in activated eosinophils. EPO consists of a heavy chain and a light chain encoded with a prosequence. The EPO gene is on chromosome 17 and maps closely to myeloperoxidase and lactoperoxidase genes, two other members of the mammalian peroxidase family found in neutrophils and mucosal glands, respectively. Although MBP is present in the highest molar concentration in eosinophil granules, EPO, by weight, is the most abundant protein constituting approximately 25% of the specific eosinophil granule’s total protein mass. EPO catalyzes the oxidation of halides, pseudohalides, and nitric oxide to form highly reactive oxygen species (hypohalous acids), reactive nitrogen metabolites (nitric dioxide), and other oxidants that then oxidize targets on proteins with oxidative stress and subsequent cell death by apoptosis and necrosis. EPO kills numerous microorganisms in the presence of hydrogen peroxide, generated by eosinophils and other phagocytes, and halide. This combination of products also initiates mast cell secretion. As noted, both EPO and MBP induce noncytolytic, dose-dependent 5-hydroxytryptamine release from platelets.82 Binding of EPO to neutrophils reversibly inhibits EPO peroxidase activity but increases neutrophil aggregation and adhesion to endothelial cells.83 EPO binding to microbes, including Staphylococcus aureus, greatly potentiates their killing by phagocytes. EPO-coated tumor cells are spontaneously lysed by activated macrophages.
Eosinophil cationic protein (ECP or RNase3) and eosinophil-derived neurotoxin (EDN or RNase2) are homologous proteins with sequence identity in 37 of 55 amino acid residues and are encoded on chromosome 14. ECP also has neurotoxic activity. ECP and EDN play a role in viral host defense to RNA viruses.51,88,89 New roles for these proteins continue to be identified.90 EDN induces the migration and maturation of dendritic cells.91 It also is an endogenous ligand of Toll-like receptor 2 (TLR2) and can activate myeloid dendritic cells by triggering the TLR2-myeloid differentiation factor 88 (Myd88) signaling pathway.60 Based on its ability to serve as a chemoattractant and activator of dendritic cells along with enhancing antigen-specific Th2-biased immune responses, EDN functions as an alarmin alerting the adaptive immune system to preferentially enhance antigen-specific Th2 responses.60
They are found in the matrices of specific granules in mature eosinophils. Both proteins also are present in mature neutrophils.84 They are highly basic proteins, the pI of ECP is 10.8 and the pI of EDN is 8.9. It is likely that these two proteins arose as a consequence of gene duplication 25–40 million years ago.85 EDN, in particular, is a rapidly evolving protein, accumulating nonsilent mutations at a rate exceeding those of most other functional coding sequences studied in primates.86 Although the homology in EDN and ECP sequences accounts for certain similarities, their differences are considerable. ECP and EDN are members of the human RNase A family, ECP is a relatively weak ribonuclease, and EDN is a potent ribonuclease. ECP is a potent toxin for parasites through a different mechanism than MBP-1 and is more effective at killing certain helminths than MBP-1. EDN, as its name implies, was discovered as the neurotoxin that accounted for the Gordon phenomenon, described in 1933, in which splenic extracts from patients with the Hodgkin’s disease caused severe damage to myelinated neurons in experimental animals.87
Distinctive hexagonal, bipyramidal crystals were initially described in 1853 in a patient with leukemia and later, in 1872, in the sputa of asthmatic patients. Since then, CLCs have been regarded as a hallmark of eosinophilia. CLC protein is an abundant, characteristic, although not unique, protein of eosinophils. It is also found in lesser amounts in basophils. CLC mRNA and EDN mRNA are among the most highly expressed mRNAs in mature peripheral blood eosinophils suggesting de novo synthesis.1
![]() CLC belongs to a family of galactose-binding lectins and also is called galectin-10.92 CLC protein is localized to primary eosinophil granules but also is detected in the nuclear matrix and cytoplasm of eosinophils derived from IL-5-treated umbilical cord cell cultures. Moreover, in cells expressing the recombinant molecular form, CLC protein is detected in the nucleus, the cytoplasm and the plasma membrane. The role of CLC protein is unknown. Previous studies suggested that it may have lysophospholipase activity or lysophospholipase inhibitor binding activity, with the theory that this activity could protect the cells from the lytic effects of lysophospholipase at sites of inflammation. However, CLC protein has no sequence similarities to known lysophospholipases, and depletion of CLC protein from eosinophil lysates did not alter their lysophospholipase activity. Further, affinity-purified CLC protein lacked lysophospholipase activity. Therefore, CLC protein and eosinophil lysophospholipases are distinct proteins.93 Lastly, through its lectin-like domains, CLC protein may bind carbohydrates expressed on microorganisms or biological molecules, such as IgE or laminin.94
CLC belongs to a family of galactose-binding lectins and also is called galectin-10.92 CLC protein is localized to primary eosinophil granules but also is detected in the nuclear matrix and cytoplasm of eosinophils derived from IL-5-treated umbilical cord cell cultures. Moreover, in cells expressing the recombinant molecular form, CLC protein is detected in the nucleus, the cytoplasm and the plasma membrane. The role of CLC protein is unknown. Previous studies suggested that it may have lysophospholipase activity or lysophospholipase inhibitor binding activity, with the theory that this activity could protect the cells from the lytic effects of lysophospholipase at sites of inflammation. However, CLC protein has no sequence similarities to known lysophospholipases, and depletion of CLC protein from eosinophil lysates did not alter their lysophospholipase activity. Further, affinity-purified CLC protein lacked lysophospholipase activity. Therefore, CLC protein and eosinophil lysophospholipases are distinct proteins.93 Lastly, through its lectin-like domains, CLC protein may bind carbohydrates expressed on microorganisms or biological molecules, such as IgE or laminin.94
![]() Many enzymes, in addition to EPO and the RNases (EDN and ECP), have been identified in human eosinophils, granules, and membranes (see Fig. 31-2).1 Arylsulfatase B, β-glucuronidase, lysozyme, acid phosphatase, catalase, and histaminase are also present in eosinophils. Eosinophils are a source of matrix metalloproteinase-9 (MMP-9), which is important for their migration through basement membranes. Eosinophil-derived MMP-9 is found in basal cell and squamous cell carcinomas95,96 and in bullous pemphigoid lesions.97 MMP-9 cleaves type XVII collagen (BP 180), a transmembrane molecule of the epidermal hemidesmosome, likely contributing to or causing basement membrane zone separation in bullous pemphigoid. Collagenase, which degrades type I and type III collagen, may play a role in tissue remodeling. Eosinophils generate extracellular superoxide anions through activation of a plasma membrane NADPH oxidase, expressed in high levels in human eosinophils. Activated eosinophils form more of the functional NADPH oxidase complex and produce more superoxide than neutrophils or macrophages.98 Secretory phospholipase A2 is mainly located in specific granules in the eosinophil and copurifies with ECP in granule fractions. It is known to degrade phospholipids of Gram-negative bacteria and to cause airway inflammation and smooth muscle contraction. Secretory phospholipase A2 may act together with bactericidal/permeability-increasing protein (BPI), also present in eosinophil granules, in microorganism defense. Cytosolic phospholipase A2 plays an essential role in mediating hydrolytic cleavage of arachidonic acids in membrane phospholipids and generating various lipid mediators (vide infra).1
Many enzymes, in addition to EPO and the RNases (EDN and ECP), have been identified in human eosinophils, granules, and membranes (see Fig. 31-2).1 Arylsulfatase B, β-glucuronidase, lysozyme, acid phosphatase, catalase, and histaminase are also present in eosinophils. Eosinophils are a source of matrix metalloproteinase-9 (MMP-9), which is important for their migration through basement membranes. Eosinophil-derived MMP-9 is found in basal cell and squamous cell carcinomas95,96 and in bullous pemphigoid lesions.97 MMP-9 cleaves type XVII collagen (BP 180), a transmembrane molecule of the epidermal hemidesmosome, likely contributing to or causing basement membrane zone separation in bullous pemphigoid. Collagenase, which degrades type I and type III collagen, may play a role in tissue remodeling. Eosinophils generate extracellular superoxide anions through activation of a plasma membrane NADPH oxidase, expressed in high levels in human eosinophils. Activated eosinophils form more of the functional NADPH oxidase complex and produce more superoxide than neutrophils or macrophages.98 Secretory phospholipase A2 is mainly located in specific granules in the eosinophil and copurifies with ECP in granule fractions. It is known to degrade phospholipids of Gram-negative bacteria and to cause airway inflammation and smooth muscle contraction. Secretory phospholipase A2 may act together with bactericidal/permeability-increasing protein (BPI), also present in eosinophil granules, in microorganism defense. Cytosolic phospholipase A2 plays an essential role in mediating hydrolytic cleavage of arachidonic acids in membrane phospholipids and generating various lipid mediators (vide infra).1