Fig. 11.1
Cytoplasmic malonyl-CoA decarboxylase assures that acetyl-CoA and methylmalonyl-CoA are the only substrate available for FAS
The cytoplasmic malonyl-CoA decarboxylase has been purified, its cDNA has been cloned and the gene that encodes it has been sequenced (Jang et al. 1989). The results showed that the gene is transcribed to produce an mRNA that would be translated into a protein with a mitochondrial targeting leader sequence in the liver (Courchesne-Smith et al. 1992). In the gland, a different transcription start site is used to generate an mRNA that would encode a decarboxylase protein that lacks the leader sequence and thus remains in the cytoplasm (Fig. 11.2). Thus, a change in the transcription initiation site in the gland causes accumulation of malonyl-CoA decarboxylase in the cytoplasm and causes the production of multiple methylbranched fatty acids using the same acyl-CoA carboxylase and fatty acid synthase present in other tissues. This was an unexpected discovery of a minimal change in gene expression that leads to a major change in the composition of the lipids produced by the specialized sebaceous gland. It is interesting to note that the malonyl-CoA decarboxylase gene sequence, first revealed by the work on the uropygial gland, was used to clone the homologous mammalian gene that turned out to be an important player in cardiovascular health by regulating fatty acid oxidation (Lopaschuk and Stanley 2006; Ussher and Lopaschuk 2008) and thus, is currently under extensive investigation (Froese et al. 2013).
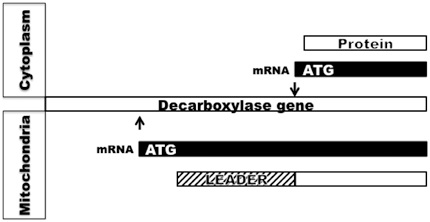
Fig. 11.2
Use of alternate transcription initiation site in the goose uropygial gland leads to an mRNA that is translated into malonyl-CoA decarboxylase protein lacking leader sequence causing accumulation in the cytoplasm. Arrows indicate transcription initiation site
It is interesting to note that mycobacteria, that produce 2,4,6,8 tetramethyl branched long-chain acids as one of the main components of its cell wall-associated lipids, has evolved a unique multifunctional synthase that uses n-C20CoA as the starter, in place of acetyl or propionyl CoA, and generate 2,4,6,8 tetramethyl tetracosanoic acid called mycocerosic acid (Rainwater and Kolattukudy 1985). In this case the acyl transferase and condensing domains of the multifunctional peptide have evolved to enable the synthase to use n-C20CoA as the starter, and methylmalonyl-CoA as the chain extending substrate to build the multiple methylbranched mycocerosic acid (Fernandes and Kolattukudy 1997). Thus, when a small specialized tissue within an animal needs to make multiple methyl branched acids, a tissue-specific switch in transcription initiation site allows the gland to use the highly evolved acyl-CoA carboxylase and FAS to produce the unusual lipids. When a whole organism, like the Mycobacterium, needs to make an unusual multiple methyl branched acid, it has evolved a new multifunctional fatty acid synthase uniquely for the synthesis of such an unusual acid.
Very Long Chain Acids
Presence of compounds with very long alkyl chains is a characteristic feature of surface lipids. The very long chain fatty acids are generated by chain elongation enzymes that are located in the membranes, primarily in the endoplasmic reticulum (ER). These enzymes use n-C16 acid that is generated by the cytoplasmic FAS as the starter that is activated by the ER-located enzyme, and then elongate the thioester using malonyl-CoA and NADPH as the other substrates using reactions analogous to those catalyzed by FAS: condensation, ketoreduction, dehydration, and enoyl reduction. These enzymes probably function as a complex attached to the membrane. Membrane preparations from sebaceous glands such as meibomian glands have been demonstrated to catalyze such elongation reactions generating very long chain fatty acids (Anderson and Kolattukudy 1985). The condensing enzyme determines the specificity of the elongating enzyme and a family of elongases, ELOVL1-7, have been identified (Kihara 2012). They show selectivity for the starting acyl-CoA they accept for elongation. ELOVL3 and ELOVL4 have been shown to play important roles in skin lipid biosynthesis (Westerberg et al. 2004; Vasireddy et al. 2007). Disruption of elovl3 gene, that shows restricted expression in the sebaceous gland and hair follicular epithelial cells, displayed a sparse hair coat with disturbed hair lipid content (Westerberg et al. 2004). Fatty acids longer than 20 carbons were virtually undetectable. Consequently, elovl3 deficient mice showed a severe defect in water repulsion and high levels of trans-epidermal water loss. Mutation in elovl4 resulted in the absence of very long chain > C28 acids and ω-O-acyl ceramide that are important components of the extracellular lamellar membrane that constitutes a major permeability barrier (Vasireddy et al. 2007; Uchida 2011). Consequently, these mice displayed scaly, wrinkled skin with severely compromised epidermal permeability barrier causing death within a few hours after birth.
Fatty acids used for the biosynthesis of skin lipids are not only generated within the skin lipid-synthesizing cells, but also transported into such cells. A family of fatty acid transport proteins (FATP) is involved in this process (Khnykin et al. 2011). Of the six such mammalian FATPs, FATP4 seems to be the most important one involved in skin lipid biosynthesis (Schmuth et al. 2005). Thus, deletion of FATP4 in mice results in perturbations in the biosynthesis of skin lipids by keratinocytes that causes barrier dysfunction and consequently neonatal fatality (Lin et al. 2013a). Keratinocyte targeted expression of FATP4 reverses these abnormalities. Wax diester synthesis is drastically reduced by the absence of FATP4. Biosynthesis of the very long chain acids is preferentially inhibited by the absence of FATP4, as the membrane localized elongating enzyme system responsible for the biosynthesis of such acids probably receives the acyl chains for elongation in the activated form from the membrane localized FATP4.
Short-Chain Fatty Acid Synthesis
Chain length of fatty acids generated by multifunctional synthases is determined by the chain length selectivity of the chain-terminating thioesterase that is a segment of FAS. The thioesterase segment of vertebrate FAS, including avian FAS, releases the acyl chains when they reach about 16 carbon length (Bedord et al. 1978). Certain avian uropygial glands, such a mallard duck gland, produce esters of shorter chain fatty acids such as C8, C10, and C12 (Jacob 1976). In such glands a small (30 kDa) thioesterase, uniquely present in the glands, interacts with the FAS and releases the shorter acyl chains from the synthase (deRenobales et al. 1980). Such a thoiesterase was purified to homogeneity from mallard uropygial glands and its cDNA cloned (Rogers et al. 1982; Poulose et al. 1985). This thioesterase reacted with pyrenelbutylmethane phosphono fluoridate with covalent attachment of the pyrene derivative to the active serine resulting in the inactivation of the enzyme (Foster et al. 1985a). Addition of avian fatty acid synthase to pyrene-tagged thiosterase caused a dramatic increase in fluorescence anisotropy of the pyrene, demonstrating the physical interaction between the thiosterase and FAS. The association constant for binding of the two proteins was calculated to be 1 µM with a one to one stoichiometry. This association could be used to conveniently purify the thioesterase from gland extracts using fatty acid synthase as an affinity ligand (Rogers and Kolattukudy 1984).
The small S-acyl-FAS thioesterase was functionally compatible with vertebrate FAS from other sources (Rogers et al. 1982). FAS from murine source and goose were inactivated by PMSF treatment that covalently modified the active serine in the chain-terminating thioesterase segment of FAS. These inactive FAS preparations were reactivated by the addition of the duck small S-acyl FAS thioesterase that released the acyl chains from FAS. Thus, it would appear that the terminal thioesterase domain of multifunctional FAS polypeptide can be functionally replaced by the small s-acyl FAS thioesterase. The interaction between the resident thioesterase domain of FAS with the fatty acid chain built on the thiol at the condensing domain was examined by determining the distance between the active sites of the thioesterase and the pantetheine thiol using Florence Resonance Energy Transfer (Foster et al. 1985b). The active serine was tagged by treatment of the synthase with pyrenebutylmethane phosphono fluoridate. The pantetheine thiol was tagged by treatment with 3- (4 meleinidylphenyl)-7- diethylamino -4- methyl coumarin. When FAS was thus tagged at the thioesterase active site with pyrene and ACP domain active site with coumarin, fluorescence energy transfer occur between the pyrene and the coumarin. The distance between the pyrene and coumarin was estimated to be 37 A from the efficiency of energy transfer. Similar results were observed when the small S-acyl-FAS thioesterase tagged with pyrene was used with coumarin-tagged FAS, supporting the conclusion that the small thioesterase physically interacts with FAS and functionally displaces the resident thioesterase of FAS.
Biosynthesis of Fatty Alcohols
Fatty acyl-CoA reductase that catalyzes fatty alcohol production was first demonstrated in Euglena gracilis in which a particulate enzyme catalyzes direct conversions of fatty acyl-CoA to fatty alcohol with NADPH as the reductant without releasing fatty aldehyde intermediate (Kolattukudy 1970). Since then fatty acid acyl-CoA reductases have been found and purified from bacteria, plants, and animals, including sebaceous glands (Hellenbrand et al. 2011; Cheng and Russell 2004a; Moto et al. 2003; Honsho et al. 2010). Microsomes from bovine meibomian gland and avian uropygial glands also catalyze reduction of fatty acyl-CoA to alcohol without release of any aldehyde intermediates (Kolattukudy and Rogers 1986). A microsomal enzyme solubilized and purified form the duck uropygial gland was found to catalyze conversion of fatty acyl-CoA to fatty alcohol without detectable free fatty aldehyde intermediate (Wang and Kolattukudy 1995a). Fatty acyl-CoA reductase that generates aldehyde as the final product was separated from an aldehyde reductase from a plant that generates both fatty alcohols and alkanes from aldehyde (Kolattukudy 1971). A fatty acyl-CoA reductase that generates aldehydes and alcohols was found in Mycobacteria that makes alcohols for wax ester synthesis (Sirakova et al. 2012). Thus, reductases that reduce fatty acyl-CoA to alcohol via free fatty aldehyde intermediate are known.
In more recent years, bioinformatics approaches have been used to identify and clone acyl-CoA reductases. Two fatty acyl-CoA reductases have been identified in human and mouse with an insilico approach (Cheng and Russell 2004a). When the cDNAs encoding FAR1 and FAR2 were expressed in cells, these proteins catalyzed acyl-CoA reduction with NADPH as the reductant. FAR1 showed a preference for C16 and C18 saturated and unsaturated acyl-CoA whereas FAR2 preferred saturated C16 and C18 acyl-CoA. FAR1 was found in a variety of murine tissue but FAR2 showed a more restricted occurrence with highest expression in the eyelid that has the wax ester-producing meibomium glands. Both FAR1 and FAR2 that use alcohols for the synthesis of ether lipids were found in the brain. cDNA encoding two fatty acyl-CoA reductases have been cloned from the uropygial glands of several bird species (Hellenbrand et al. 2011). The products of these reductases, FAR1 and FAR2, revealed some differences in substrate specificity. Avian FAR1 showed highest expression in the uropygial gland whereas the avian FAR2 was highly expressed in the brain that is rich in ether lipids.
Biosynthesis of Diesters
Five types of diesters are found in sebaceous secretions. The two most common diesters produced by animal sebaceous glands are 2-hydroxy acids esterified with a fatty alcohol and a fatty acid, and alkane-1,2-diol with both hydroxyl groups in ester linkage with fatty acids (Downing 1976). Diesters of alkane α, ω-diols found in the mammalian meibomian gland constitute a third type of diesters (Nicolaides and Santos 1985). A fourth type of diesters, 3-hydroxyfatty acids with both the hydroxyl group and carboxyl group in ester linkages with a fatty acid and fatty alcohol, respectively, are produced as the major secretion product of mallard duck uropygial gland during the mating season (Kolattukudy et al. 1987a). A fifth type of diester alkane-2,3-diol diesters are the major secretion products of the uropygial glands of galliformis, such as chicken, quail, pheasant, etc. (Jacob 1976).
α-Hydroxylation of fatty acids to generate α-hydroxy fatty acid was found to be catalyzed by a particulate fraction from the uropygial glands of a white-crowned sparrow, Zonotrichia leucophrys (Kolattukudy 1972). The same particulate preparation also catalyzed α-hydroxyfatty acyl-CoA reduction to generate alkane-1,2-diol with NADPH as the reductant with hydride transfer from the B-side of the nicotinamide ring. Mammalian sebaceous glands also produce diesters that require 2-hydroxylation of fatty acids followed by reduction of the activated carboxyl group to generate alkane-1,2-diols (Fig. 11.3). 2-Hydroxylated fatty acid containing sphingolipids are also abundant in mammalian skin. Mammalian fatty acid 2-hydroxylase (FA2H) is an NADPH dependent enzyme that catalyzes stereospecfic hydroxylation to yield R-enantiomer (Maier et al. 2011; Guo et al. 2012). When FA2H gene, that was thought to be important for the synthesis of hydroxylated fatty acid containing sphingolipids of the murine skin, was knocked out, the FA2H deficient mice did not show any effect on the level of such sphingolipids (Maier et al. 2011). FA2H expression was found to be restricted to sebaceous glands and FA2H deficiency caused a drastic reduction in 2-hydroxylated glucosylceramides and in diester waxes. The consequent change in composition of the surface lipids caused a blockage in hair canals and thus severely interfered with hair growth.
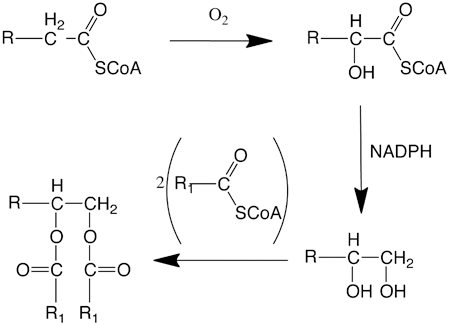
Fig. 11.3
Biosynthesis of Alkane-1,2-diol diesters
Alkane-α, ω-diols probably arise by ω-hydroxylation of a very long fatty acid followed by a reduction of the activated carboxyl group by a reductase. Biosynthesis of the esters containing very long chain ω-hydroxyfatty acid present in meibomian gland (Kolattukudy et al. 1985a) and the very long chain ω-hydroxyceramides that are highly significant constituents of the extracellular multilamellar bodies in the outer layer of the skin involve ω-hydroxylation by cytochrome P450 (CYP) (Hardwick 2008). Among the super family of CYPs, CYP4F8, and CYP4F22 have been implicated in skin lipid biosynthesis (Kelly et al. 2011). CYP4F8 is expressed in the mammalian epidermis and its expression level is elevated in psoriasis (Stark et al. 2006). Mutations in CYP4F22 gene were found in patients with ichtyosis with abnormality in permeability barrier due to deficiency in very long chain acids and absence of ω-O-acylceramides (Lefèvre et al. 2006). Deficiency in cytochrome b5 that is an electron transport mediator involved in ω-hydroxylation also results in compromised permeability barrier, probably by a combination of changes in unsaturated fatty acid synthesis and a reduction in ω-hydroxylation required for the biosynthesis of the constituents of the barrier (Finn et al. 2011).
Diesters of 3-hydroxy C8, C10, and C12 acids are produced by female mallard ducks during the breeding period (Kolattukudy and Rogers 1987). A particulate fraction from the gland extract was shown to catalyze 3-hydroxy-C12 fatty acyl-CoA synthesis. The 3-hydroxy fatty acids are produced by hydration of enoyl-CoA generated by peroxisomal fatty acyl-CoA oxidase as shown by biochemical experimental evidence (Bohnet et al. 1991; Fig. 11.4). As indicated later, these processes occur in peroxisomes that proliferate in the female duck uropygial gland during the mating season.
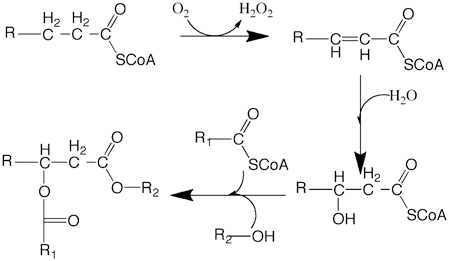
Fig. 11.4
Biosynthesis of 3 hydroxyfatty acid diesters
Biosynthesis of alkane-2,3-diol was postulated to involve condensation between fatty aldehyde and the thiamine pyrophosphate derivative of acetaldehyde to generate an acyloin that could undergo reduction to alkane-2,3-diol (Fig. 11.5; Sawaya and Kolattukudy 1972). Results obtained with chicken uropygial glands were consistent with this hypothesis (Tang and Hansen 1976). The uropygial gland of ring-necked pheasant (Phasianus colchicus), produces octadecane-2,3-diol as the major diol (85 %). Location of the label in the diol derived from [1-14C] palmitic acid in the gland at C-3 of octadecane-2,3-diol was consistent with the proposed biosynthetic pathway (Sawaya and Kolattukudy 1972). Furthermore, synthetic 3-hydroxy-[3-14C]-octadecane-2-one, the predicted acyloin intermediate, was converted directly into octadecane-2,3-diol when injected into the uropygial gland of the pheasant (Buckner and Kolattukudy 1976b). A cell-free preparation from the gland catalyzed the transfer of hydride from the B-side of the nicotinamide ring of NADPH to the carbonyl carbon of synthetic R,S mixture of the acyloin to generate a mixture of threo- and erythro-octadane-2,3-diol.
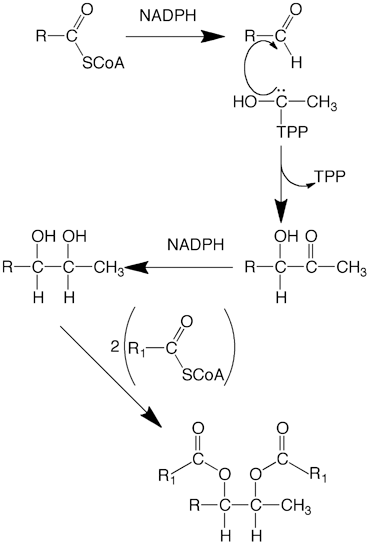
Fig. 11.5
Biosynthesis of Alkane-2,3-diol diesters
Esterification
We have come a long way from the days when direct esterification of a free fatty acid with fatty alcohol was thought to be the mechanism of wax ester synthesis (Friedberg and Greene 1967). With plant enzymes it was demonstrated for the first time that fatty acyl-CoA is used to esterify fatty alcohol (Kolattukudy 1967) (Fig. 11.6). It has been shown that particulate preparations from the avian uropygial glands and mammalian meibomian glands catalyze esterification of fatty alcohols, alkane-1,2-diols, alkane-2,3-diols, 2-hydroxyfatty acids, and 3-hydroxyfatty acids using acyl-CoA (Sawaya and Kolattukudy 1973, Cheng and Russell 2004b; Turkish et al. 2005; Biester et al. 2012). cDNA for wax ester synthases from human, mouse, and avian sources have been cloned and their expression products examined (Cheng and Russell 2004; Miklaszewska et al. 2013). Wax synthase was first cloned from jojoba embryo and expressed in arabidopsin with the demonstration of abundant wax ester synthesis in the seeds of transgenic plants (Lardizabal et al. 2000). Wax synthase-encoding sequences in animals were first identified by expression cloning of cDNA from mouse preputial glands (Cheng and Russell 2004b). This enzyme showed esterifying activity with a variety of alcohols, including polyisoprenol, and acyl-CoA, but did not show ability to acylate cholesterol or monoacylated or diacylated glycerol. Members of DGAT1 and DGAT2 families of acyl transferases from mammalian and avian sources catalyze acylation of alcohols, diols, retinol, etc. as well as a diacylglycerol. For example, human DGAT1 can catalyze formation of monoesters, diesters, and retinyl esters (Yen et al. 2005a; Yen et al. 2005b). Human wax synthases AWAT1 and AWAT2, belonging to DGAT2 family, can transfer acyl groups to mono or diacylglycerol, fatty alcohol and retinol (Turkish and Sturley 2009). Most probably, the multifunctional acyl tranferases can account for the synthesis of mono- and diester waxes. The presence of transmembrane domains in the wax synthase explains the membrane localization of the acylating enzyme activity in the cell. In cell homogenates however, the nature of the esterifying enzymes catalyzing these reactions have not been elucidated in many cases. Whether specific acyltransferases are involved in the esterification to produce each type of ester remains to be established. However, the possibility of transacylation to diacyl glycerol, fatty alcohol and alkane-1,2-diol by the same enzyme has been strongly suggested. Two acyl transferases encoded by two separate genes catalyze acylation of diacylglycerol to yield triacylglycerol. Of these, DGAT2 seems to be specializing on triacylglycerol synthesis whereas DGAT1 can catalyze acylation of not only diacylglycerol but also alkanol, alkane-1,2-diol and retinol using fatty acyl-CoA as a substrate (Yen et al. 2005a). Deletion of DGAT1 gene in mice shows abnormalities in skin lipid composition with complete absence of diol diesters reflecting its in vivo role in acylation reaction involved in skin lipid biosynthesis. Whether such multifunctional acyl transferases are involved in the synthesis of esters in sebaceous glands in other species remains to be established.
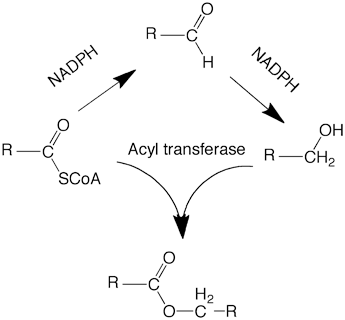
Fig. 11.6
Biosynthesis of monoester wax
Biosynthesis of Alkanes
Presence of alkanes as a major component of sebaceous gland lipids is not common, and the alkanes reported to be present in animal surface lipids are thought to be of exogenous origin. However, uropygial gland lipids of a water fowl, eared grebe (Podiceps nigricollis), contain large amounts (35–40 %) C21, C23, C25 and C27 alkanes (Cheesbrough and Kolattukudy 1988).
Mechanism of biosynthesis of alkanes, the simplest organic compounds, remained unknown for a long time. In plants, very long chain alkanes are present in significant amounts, together with ketones and secondary alcohols with the functional group in the middle of the carbon chain. This prompted the proposal that head to head condensation between two fatty acids would lead to the formation of the ketone that would give rise to secondary alcohols and ultimately to alkanes. Experiments with 14C-labeled precursors disproved this hypothesis and showed that chain elongation of fatty acids followed by the loss of the carboxyl carbon would give rise to long chain alkanes (Kolattukudy 1987). The mechanism by which the carboxyl carbon of a fatty acid is lost remained a mystery for a long period, as direct decarboxylation of an alkanoic acid seemed mechanistically unlikely. The first clue about a possible mechanism came from the finding that inhibition of alkane synthesis by dithioerythritol resulted in the accumulation of aldehydes with one carbon more than the alkanes. The aldehydes were then shown to be the immediate precursors of alkanes. In alkane producing tissues an acyl-CoA reductase that generates aldehydes has been found (Kolattukudy 1971; Vioque and Kolattukudy 1997; Wang and Kolattukudy 1995b; Lin et al. 2013b). Decarbonylation, a novel biochemical reaction, first discovered in plants (Kolattukudy 1987; Cheesbrough and Kolattukudy 1984; Dennis and Kolattukudy 1992), was shown to be catalyzed by particulate preparations from the uropygial glands of eared grebe that converted an aldehyde to an alkane releasing CO as the other product (Fig. 11.7) (Cheesbrough and Kolattukudy 1988). Even though loss of the carbonyl carbon of an aldehyde (decarbonylation) to yield an alkane has been demonstrated in animals, insects and plants including alga (Kolattukudy 1987; Yoder et al. 1992; Schirmer et al. 2010; Qiu et al. 2012; Das et al. 2011), the chemical form of the lost carbonyl carbon (CO, CO2 or formate) and the mechanistic details of how the carbonyl carbon is removed remain to be firmly established.
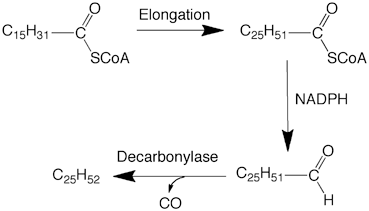
Fig. 11.7
Elongation decarbonylation mechanism for the biosynthesis of alkanes
Regulation of Wax Ester Biosynthesis in Sebaceous Glands
Developmental Changes
There are compositional changes in sebaceous gland lipids that suggest developmental changes. The differences in chemical composition of the sebaceous gland secretion found in newborn babies and those of adult humans suggest developmental changes in the synthesis of sebaceous gland lipids. As humans age the squalene and diester wax content of the sebum decreases (Strauss et al. 1975). Analysis of sebrum production in humans of different ages showed a range of levels with a general pattern of decrease with age with some differences in the level and composition between males and females (Jacobsen et al. 1985; Nazzaro-Porro et al. 1979). The chain lengths and diastereoisomer composition of alkane-2,3 diol diesters of the chicken uropygial glands change significantly as the birds become physiologically mature (Kolattukudy and Sawaya 1974). In developing embryonic goose uropygial glands malonyl-CoA decarboxylase transcripts, a major player in the synthesis of the goose uropygial gland lipids, appears several days prior to hatching and reaches maximal levels by hatching (Kolattukudy et al. 1987b).
Duck uropygial gland provides a system that illustrates developmental changes and hormonal influence on the composition of sebaceous gland wax esters. In the developing duck embryonic uropygial glands, malic enzyme and fatty acid synthase transcripts increase dramatically several days before hatching (Goodridge et al. 1984). The uropygial gland lipids in 2- to 21-day-old ducklings show a composition very different from that of the adult (Kolattukudy et al. 1991). The major components of the duckling glands are long-chain wax esters. As the ducklings approach adulthood, shorter chain esters increase. At 23 days of age shorter chain (< C12) constitute only about 5 %of the acids in the wax ester, whereas the content of such short-chain acids approach 90 % of the acids in 50-day-old ducks. Until juvenile feathers begin to appear at about 3 weeks of age the waxes are composed of extremely complex mixture of n-, mono- and dimethyl branched acids with no obviously dominant components. The complexity decreases with the appearance of adult feather patterns. Monomethyl-branched acids become the dominant acids constituting 94 % of the acids in the wax. These compositional changes, that occur during the period when the down of hatchlings is being replaced with adult feathers, is reflected in the changes in the level of the key enzyme uniquely involved in short-chain acid synthesis. S-Acyl FAS thioesterase that releases the shorter acid from FAS increases dramatically, both at the transcript level and protein level (Kolattukudy et al. 1985b) whereas the FAS level remains relatively constant.
Seasonal Changes in the Adults
Male Mallard
Immediately after the discovery of acyl-FAS thioesterase, we purified this enzyme. During a period in this effort we encountered repeated failures to get enzyme from duck gland extracts for reasons unknown to us at that time. This period turned out to be the postnuptial molt-period called eclipse. During this period the male mallards lose their colorful plumage that becomes replaced with a dull looking plumage giving the males a drab female-like appearance. Thus, we suspected that the difficulty in purifying the enzyme might be related to the physiological changes accompanying eclipse. A systematic examination of the uropygial gland lipid composition of the male mallard ducks over an entire year revealed dramatic seasonal changes in the uropygial gland lipid composition (Kolattukudy et al. 1985c). From February until April, 50–90 % of the wax acids have short chains. In May this percentage began to drop and reached less than 5 % in June and July and then recovered to > 50 % by August. These changes in short-chain acid content was found to be reflected in the S-acyl FAS thioesterase level that dropped to about 10 % during the eclipse period (July) (Kolattukudy et al. 1985c). The S-acyl-FAS thioesterase is a key regulator of the lipid composition in the duck uropygial glands. During eclipse thioesterase level drops, at transcript level and protein level. Transcription is regulated at least in part by steroid hormones. Estradiol and corticosteroid receptor binding consensus sites are present in the first intron of the thioesterase gene (Sasaki et al. 1988). During eclipse estradiol is at a maximal level in the duck. Administration of estradiol or dexamethasone, a synthetic steroid that is more potent than cortisol, reduced the thioesterase transcript and protein levels in the gland (Sasaki and Kolattukudy, unpublished results). Nuclear run off experiments showed that the rate of transcription of thioesterase gene is lower (~ 10 %) in nuclei from glands from animals treated with either estradiol or dexamethasone. This suppression of thioesterase gene transcription results in a drastic decrease in short chain esters in the gland. In fact, the wax ester composition of estradiol or dexamethasone-treated animal glands is very similar to the composition found during eclipse (Fig. 11.8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






