This article discusses the role of injectable soft-tissue fillers in the aging face, and their clinical and chemical behavior. Temporary and permanent fillers are discussed, namely hyaluronic acids, calcium hydroxylapatite, poly- l -lactic acid, liquid silicone, and polymethylmethacrylate. Techniques and outcomes are presented.
- 1.
An array of injectable soft tissue fillers are now approved by the U.S. Food and Drug Administration (FDA). A complete understanding of their physicochemical properties and performance in clinical trials will aid in product selection and determining accurate volume requirements.
- 2.
More robust fillers are used for deeper wrinkles, folds, and cheek or chin augmentation, whereas softer and less robust products, particularly hyaluronic acid (HA) products, are used for softer depressions and fine lines, periocularly, and for the lips. Often the best results occur with a combination of fillers.
- 3.
Surgeons should use aesthetic responsibility when devising an injection treatment plan, and properly select fillers from a palate. Rather than focusing on one isolated cosmetic unit, a pan-facial approach is often best to create or restore proportion and harmony. Overvolumizing or exaggerating the proper proportions of the lips or cheeks should be avoided.
Hyaluronic acids
As a person ages, the amount of HA in the skin is reduced, decreasing the skin’s water-binding capacity and tissue turgor, leading to visible wrinkles and drooping skin. Degradation of HA is accelerated with sun exposure and aging. Injectable HA fillers are designed to restore the appearance of youth to the skin through replacing HA and binding water, thus reducing the appearance of sagging skin and skin folds.
Currently four HA fillers are FDA-approved: Juvederm Ultra and Ultra Plus (Allergan Inc, Irvine, CA, USA), Restylane/Perlane (Medicis, Scottsdale, AZ, USA), Prevelle Silk (Mentor, Irving, TX, USA), and Elevess (Anika Therapeutics, Woburn, MA, USA). Belotero (Merz, San Mateo, CA, USA) is expected to receive FDA approval shortly. Juvederm Voluma is currently under FDA review.
Several variables affect the performance of individual HA fillers, including the concentration of HA, degree of cross-linking, cohesivity, G′ (elastic modulus), and particle size, which all interact to create the unique properties of a particular HA product. An understanding of these basic science variables will help readers differentiate among available products.
One of the most important determinants of the degree of correction obtained is the HA concentration, which is not a straightforward measurement. HAs are linear polysaccharide chains that must be chemically cross-linked to be stable in vivo. The concentration of HA, measured in milligrams per milliliters, includes both cross-linked HA and free (non–cross-linked or soluble) HA, which is rapidly absorbed in vivo. Non–cross-linked HA is added to the various HA fillers as a lubricant to ease product flow through the needle, yet adds nothing to the final correction. It is best to think of concentration in terms of “effective HA concentration” (effective HA [EFA] = total HA – uncross-linked HA), which is a better measure of the HA that will contribute to tissue correction. The HA concentration also has important implications for long-term correction and initial reaction on injection. The hydrophilic nature of HA means that the more concentrated products will tend to imbibe more water, and thus have more tissue swelling after injection. After steady state equilibrium is reached with the surrounding tissue, more concentrated products will maintain more swelling and have more fullness in the area injected. Prevelle Silk, Restylane, Belotero, Juvederm, and Elevess contain 5.5, 20, 22, 24, and 28 mg/mL of HA, respectively.
When a dermal filler is implanted into or under the skin, the skin’s natural elasticity or tension will tend to flatten out the implant, reducing the initial desired correction. The force of the filler that opposes and resists this tension determines the “lift capacity” of a dermal filler. Although complicated and multifactorial, lift capacity is partly directly related to two material properties of the HA gel, namely the elastic (also known as storage) modulus or G′ (pronounced G-prime ), and cohesivity. The lift capacity of an HA filler increases with higher values of both G′ and cohesivity. G′ is measured through placing a specific HA product between two metal plates (a parallel plate rheometer). The amount of resistance encountered by the top plate as it slides over the gel determines the G′ for that gel and is affected by the degree of cross-linking between the chains, total HA concentration, and particle size and shape. More heavily cross-linked products tend to be stiffer and have a higher G′, and are more difficult to push through a needle. Often, HA products with a high G′ will have higher amounts of free HA to serve as a lubricant and ease the product flow through small needles. Cohesivity, on the other hand, is related to a specific HA gel’s ability to retain its shape on injection. A higher cohesivity value represents a higher resistance to deformation of the product. Of the products currently available in the United States, Prevelle Silk has the lowest concentration and lift capacity. Restylane, Juvederm, and Belotero products have a similar HA concentration and similar lift capacities, although the Restylane family, compared with the Juvederm family, relies on a higher G′ to achieve lift, whereas Juvederm relies on higher cohesivity.
During manufacturing, HA gels are produced in large gelatinous blocks of cross-linked material. Once the gel blocks are manufactured, they must be reduced in size to pass through a syringe and needle. Pushing the gel block through a “screen” mechanism produces Restylane and Perlane, so that the final particles are of a similar size with standardized shapes. It has been hypothesized that the larger particles found in Perlane have a smaller surface to volume ratio that conveys longer duration of correction because of resistance to enzymatic breakdown by hyaluronidase. However, contradicting this is the fact that both Restylane and Perlane (which has larger-sized particles) have the same duration of correction in the nasolabial crease. This property is believed to be from the porous nature of the particles that negates the surface area effects. A second way of sizing the gel block is homogenization , which is used for the Juvederm family of products. Homogenization results in particles that are variable in size, and is partially responsible for the lower G′ and extrusion force of the products. Belotero relies on a cohesive polydensified matrix that contains zones of HA that have different degrees of cross-linking, which results in a continuous gel that does not undergo particle formation before injection.
The most important aspect of injectable HAs is how they behave clinically. Prevelle Silk is a 5.5-mg/mL HA particle gel containing lidocaine, which decreases pain on injection. Compared with Restylane and Juvederm, it contains a much lower concentration of HA and is considered a “softer” HA filler with less lift capacity, and has an average duration of correction of approximately 3 months. Because it is maximally saturated with water, it undergoes little swelling on injection, which limits edema, redness, and bruising. It is a niche filler for finer lines and for patients who desire a minimum of downtime and are willing to sacrifice duration of correction.
The pivotal FDA trials and extended-duration trials for Restylane, Juvederm, and Belotero all have produced similar data. Despite differences in G′, cohesivity, concentration, cross-linking, and amounts of free HA, all three products seem to be capable of producing a similar result in the nasolabial fold (NLF). Specifically, randomized, split-face, double-blinded trials comparing each product with Zyplast collagen show that each product is superior to Zyplast at 6 months. Furthermore, each of these fillers seems to be capable of achieving corrections that persist for up to a year or longer, particularly after repeat treatment. Extended-duration studies using repeat treatment with each product show that when given 6 months or more after initial optimal correction, less product is required to maintain optimal correction and that duration of correction becomes progressively longer with each retreatment. This finding may be because of accumulation of product or HAs ability to produce neocollagenesis on fibroblast stretching. Proponents of Juvederm posit that the gel is smoother and more cohesive, with less swelling on injection, particularly in the lips. Proponents of Belotero cite histologic studies to argue that injection with Belotero results in a more even distribution of HA within the dermis and less inflammation on injection, resulting in less edema and redness, and that it is a product that performs well in fine lines without producing a Tyndall effect. These claims remain to be proven in well-designed head-to-head clinical trials comparing each product directly. Juvederm and Restylane are now available with admixed lidocaine, which has been proven to significantly reduce pain on injection.
Elevess is a 28-mg/mL HA filler that contains admixed lidocaine. It also contains sodium metabisulfite as an antioxidant and is contraindicated in patients with sulfite allergies. The FDA pivotal trial was a prospective, randomized, double-blinded, multicenter study comparing Cosmoplast with Elevess. Results proved that, compared with Cosmoplast, less Elevess was required to achieve optimal correction. Compared with Cosmoplast, Elevess showed a statistically significant greater improvement in correction at 4 months but not at 6 months. Adverse events were similar, except that more bruising and swelling occurred with Elevess than with Cosmoplast. Anecdotal reports suggested that inflammatory reactions might be more common with Elevess, which may be because of its high concentration and cross-linking.
All currently FDA-approved HAs have only been studied in NLFs, and carry the specific indication on package inserts that they are approved for dermal injection for correction of moderate to severe facial wrinkles and folds, such as NLFs. Although not specifically FDA-approved, available HAs have been studied off-label for correction of glabellar rhytides, oral commissures (meilolabial folds), lips, mid-face volumizing, infraorbital or nasojugal grooves (tear troughs), and augmentation of the dorsal hand.
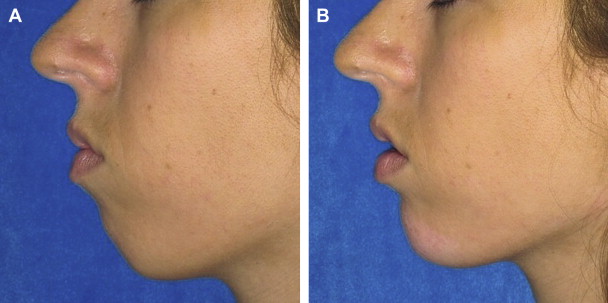
Many signs of aging are caused by loss of subcutaneous fat in the malar and submalar cheek regions. Fillers, including HAs, may successfully volumize this area when injected appropriately. Juvederm Voluma is a newer category of injectable HA intended for midface volumizing when a high lift capacity and larger volumes are required. It incorporates HA that is of lower molecular weight (representing shorter chain lengths of HA), which allows for more effective cross-linking and results in a more viscous product with a robust lift capacity. Voluma is approved for use in Europe, Canada, and Australia. It is not yet FDA-approved, although FDA studies are underway. It is a 20-mg/mL HA of streptococcal origin with a higher lift capacity. It has a lower molecular weight and a higher cross-linking ratio than other available HAs. It will be indicated for subcutaneous/supraperiosteal injection for facial volumizing and contouring ( Figs. 1 and 2 ).
Fillers must be injected in the subcutaneous or supraperiosteal plane when volumizing the mid-face. Intradermal or too-superficial injection may create persistent dermal contour irregularities. A recent study performed by Raspaldo assessed effectiveness and safety of Voluma in maintaining increased volume in the malar area for up to 18 months posttreatment. Retrospective record data were analyzed for 102 patients (93 women, 9 men; mean age, 51.27 years) who received Voluma injected into the midface. All patients were assessed at baseline and 1 month, and 6 to 18 months postinjection. The Investigator Global Aesthetic Improvement assessment after 1 month and 6 to 18 months showed that most patients were “much” or “very much” improved. Investigator volume loss assessment confirmed that most patients were either stage 1 or 2 (normal or slight mid-facial atrophy) at 1 month posttreatment, which was maintained at 6 to 18 months. Patient efficacy assessment was “very good” or “good” in most cases. The study concluded that Voluma provides aesthetic improvements according to investigator and patient assessment for up to 18 months posttreatment, with an excellent safety profile. Other studies have also documented excellent results with Voluma for age-related and HIV-related mid-facial lipoatrophy.
The safety profiles of currently FDA-approved HA fillers are good. The most common procedure- or device-related events are injection-site erythema, swelling, pain, and bruising, which all usually resolve within a few days. More serious complications can sometimes occur, but most can be avoided with appropriate injection techniques. Inappropriate and superficial placement are among the most frequent reasons for patient dissatisfaction. Too-superficial placement of HA in the dermis can result in a Tyndall effect, which is a blue discoloration caused by the refraction of light from the clear gel visible superficially in the skin. To avoid superficial injection, the metal barrel of the needle should not be visible through the skin in the plane of injection when injecting in a linear fashion.
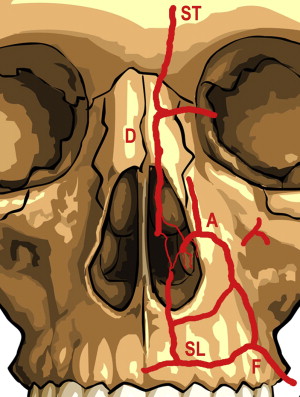
True hypersensitivity to injectable HA is rare, and occurring approximately 1 in 5000 cases. Infection is also uncommon and can usually be managed with either antibiotics or antivirals, depending on the clinical features. Injection of HA into the perioral area can potentiate recurrence of herpes simplex virus (HSV), and patients prone to recurrent perioral HSV should receive appropriate antiviral prophylaxis before treatment. The most worrisome complication is cutaneous necrosis, which is most commonly caused by occlusion of vascular structures through inadvertent injection of HA intravascularly, or through sidewall compression of vascular structures from overvolumizing of the surrounding soft tissue. The supratrochlear artery in the glabellar area and the angular artery in the superior NLF are particularly susceptible, and these areas should be considered high risk. The injecting physician should have masterful knowledge of vascular structures in areas of injection ( Fig. 3 ). A protocol to treat the full spectrum of cutaneous necrosis was recently reviewed by Hirsch and colleagues.
Because of the reversibility of HA, complications from these fillers can be easily corrected. The use of ovine testicular hyaluronidase (Vitrase) can dissolve injected HA, which is highly useful if the product is misplaced, if a complication occurs postinjection (eg, vascular occlusion, delayed granulomatous reactions) or if there is impending vascular necrosis. A recent in vitro study proves that more hyaluronidase is required to dissolve Juvederm than Restylane. In the author’s experience, 10 units of hyaluronidase per 0.1 mL of Juvederm or 5 units per 0.1 mL of Restylane to be dissolved is the most appropriate dose ( Fig. 4 ). The need for the greater amount of hyaluronidase for Juvederm is probably because the product is more highly cross-linked and the randomly shaped particles are more cohesive.
Calcium hydroxylapatite
Calcium hydroxylapatite (CaHA), marketed as Radiesse (Merz/BioForm, San Mateo, CA, USA) is a normal component of human bone and teeth and has been used as implant or coating material in dentistry and other therapeutic areas for more than 20 years. The filler is composed of CaHA microspheres (25–45 μm) suspended in an aqueous carboxymethylcellulose gel carrier. As the gel is phagocytized, the process of neocollagenesis begins in and around the microspheres, stimulating the gradual growth of the patient’s own collagen. The spherical CaHA particles are gradually broken down and degraded via normal metabolic processes, and eliminated as calcium and phosphate ions through the urinary system. The proliferation of collagen along with the slow breakdown of the CaHA is understood to account for the product’s prolonged effects lasting a year or more. It is approved for the treatment of moderate to severe wrinkles and folds, including NLFs, and correction of HIV-associated facial lipoatrophy. It is also indicated for vocal fold insufficiency, oral/maxillofacial defects, and radiographic tissue marking. Off-label facial uses also include correction of marionette lines and oral commissures, the prejowl sulcus, midface volume loss, dorsal nasal deformities, and chin augmentation. CaHA is not appropriate for use in the lips. Skin testing is not required.
CaHA was compared with a human collagen product in a United States pivotal trial of 117 subjects with moderate to severe NLFs. These patients were randomized to receive CaHA on one side of the face and an existing human collagen product (Cosmoplast, Inamed, Santa Barbara, CA, USA) on the other. CaHA provided significantly longer correction than human collagen, with 94.6% of folds graded improved, much improved, or very much improved, compared with 2.7% for human collagen. The adverse event profile was similar to that of human collagen. Adverse events were limited to erythema, edema, and ecchymosis. Edema and bruising were more common on the CaHA-treated sides than those treated with human collagen ( P <.0001). Edema and bruising lasted approximately 1 week after any injection, and the average duration for erythema was 2 to 3 weeks, with no significant difference between the materials. One nongranulomatous nodule was observed with CaHA versus three with human collagen. All adverse events resolved without sequelae. Another found longer-lasting results and increased satisfaction with CaHA compared with two HA products.
A recent study examined whether the addition of anesthetic agents (such as lidocaine) to prefilled CaHA syringes might provide sufficient anesthetic prophylaxis to reduce the need for conventional nerve blocks. The study showed that lidocaine can be added to CaHA syringes safely without harmful changes in the physical properties of the original soft tissue filler. Admixing lidocaine into CaHA at treatment was recently FDA-approved and is described on the package insert.
Radiographic studies have found that CaHA is not consistently evident on x-ray but is clearly visible on CT scans. However, CaHA is unlikely to be confused with usual abnormal and normal radiographic findings. Although usually visible on CT scans, its appearance is distinct from surrounding bony structures, does not obscure underlying structures, and does not interfere with normal analysis.
CaHA should be injected in small amounts in a retrograde fashion into the immediate subcutaneous plane or epiperiosteal plane, using a linear retrograde tunneling technique. Crosshatched linear threading may be also used. Overcorrection should be avoided. The nondominant index finger should be used to guide the needle, and the thumb and forefinger used to mold the product and remove any contour irregularities. CaHA should be injected very slowly in long, linear microthreads of approximately 0.05 mL per pass. Extreme caution should be taken when injecting into the subdermal plane around the superior NLF, where the angular artery and branches are present. Occlusion of this vessel can occur via external compression from CaHA or through injection of CaHA directly into the lumen of the vessel, creating embolic ischemia and tissue necrosis of the nasal alar region along the distribution of the angular arteries or its branches. Reports of alar vascular necrosis have been reported to the author, and the superior NLF should be considered a high risk area not only for CaHA but also for all injectable fillers. Care should also be taken not to inject CaHA epiperiosteally near the infraorbital nerve, because cases of prolonged anesthesia and paresthesia in the distribution of the infraorbital nerve have occurred with this approach.
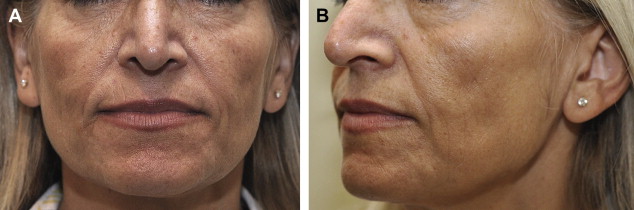
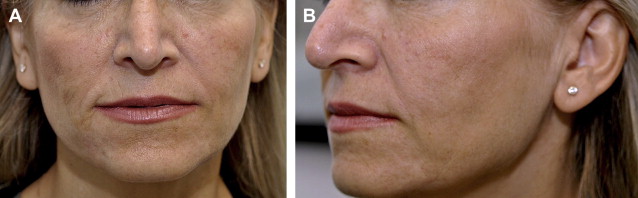
CaHA is an excellent cheek, midface, and chin volumizer ( Fig. 5 ). It is particularly important to use a sufficient volume of CaHA to treat HIV-related lipoatrophy and more advanced stages of facial lipoatrophy associated with age or lean body mass. Although previous studies have shown different efficacy end points, such as photographic documentation of global improvement and change in mean skin thickness using ultrasound or skin calipers, treatment of HIV facial lipoatrophy often falls short of optimal correction in clinical practice. In a recent study on CaHA for HIV facial lipoatrophy, the authors defined optimal correction as “very much improved” on the Global Aesthetic Improvement Score (GAIS) scale (indicating a touch up is not required) and sought to determine the volume necessary to achieve optimal correction. Using a mean cumulative volume of 13.4 mL of CaHA, 80% of patients in this study achieved the top GAIS score of “very much improved” at 3 months and 59% did so at 6 months, compared with 26% at 3 months and 7% at 6 months in a similar study that used only a mean cumulative volume of 8.4 mL of CaHA.