CHAPTER 53 Venous Thromboembolic Disease after Total Hip Arthroplasty
KEY POINTS
 Patients are at high risk for developing thromboembolic complications following total hip arthroplasty and subsequently require prophylaxis.
Patients are at high risk for developing thromboembolic complications following total hip arthroplasty and subsequently require prophylaxis. Dose-adjusted warfarin, low-molecular-weight heparins, and fondaparinux are each effective in reducing the risk of thromobembolic events following total-hip arthroplasty.
Dose-adjusted warfarin, low-molecular-weight heparins, and fondaparinux are each effective in reducing the risk of thromobembolic events following total-hip arthroplasty. While the exact duration of chemoprophylaxis is unknown, existing data supports the use of these agents for at least 10 to 14 days.
While the exact duration of chemoprophylaxis is unknown, existing data supports the use of these agents for at least 10 to 14 days.PATHOGENESIS
Multiple perioperative factors place the patient undergoing THA at risk for developing lower extremity venous thrombi. Thrombogenesis can often be related to Virchow’s triad of venous stasis, endothelial injury, and hypercoagulability. Each of these risk factors is present during the sequential stages of THA. Hip dislocation and positioning of the lower extremity for canal preparation and stem insertion result in obstruction of femoral venous outflow and subsequent stasis in the lower extremity.1 Regional edema and decreased patient mobilization after surgery may also contribute to decreased venous return. Extremes of internal rotation during positioning of the lower extremity have the potential to compress the femoral vessels and initiate secondary endothelial injury, and heat release from the use of exothermic bone cement may cause additional damage to the endothelium.1a Finally, a relative hypercoagulable state can develop during THA. Blood loss during the procedure can result in reduced serum levels of antithrombin III (AT III) and inhibition of the fibrinolytic pathway. Moreover, canal preparation and stem insertion have been shown to result in increased serum levels of markers of thrombus generation including prothrombin F1.2, thrombin-antithrombin, fibrinopeptide A, and D-dimer.2 Collectively these data suggest that initiating events that stimulate thrombus formation arise during surgery, and therefore the true goal of any prophylactic agent is to prevent further clot formation and propagation.
EPIDEMIOLOGY
Thromboembolic disease is the most common complication after THA and is ultimately responsible for more than 50% of the postoperative mortality associated with this procedure.3 In the absence of prophylaxis, asymptomatic deep venous thrombosis may develop in 40% to 60% of patients and proximal thrombosis in 10% to 40% of patients after THA (Table 53-1).4,5 The majority of these thrombi remain clinically silent and resolve without detection or further sequelae. However, a small number of patients undergoing THA (2% to 5%) will experience symptoms related to thromboembolic disease.4 Untreated thrombi have the potential to migrate proximally and in some cases embolize to the pulmonary circulation. With the shortened duration of hospital stays, symptomatic thromboembolism often manifests after discharge from the acute care setting.6 In one study approximately 20% of patients undergoing major joint surgery who had a negative venogram at discharge developed venous thrombosis over the subsequent 3 weeks.6 Other studies have suggested that although the cumulative incidence of symptomatic deep venous thrombosis was low after THA, the majority of symptomatic events (76%) occurred after hospital discharge (Fig. 53-1A).2,7
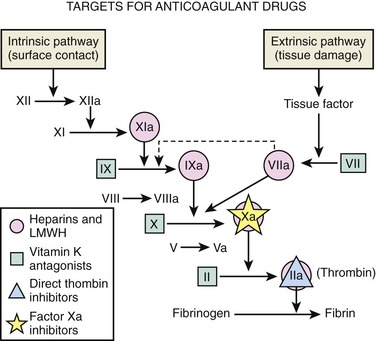
FIGURE 53-1 Targets for anticoagulant drugs. LMWH, low-molecular-weight heparin.
(Reproduced with modification from Petitou M, Lormeau JC, Choay J: Chemical synthesis of glycosaminoglycans: New approaches to antithrombotic drugs. Nature 350(Suppl):30-33, 1991. Reprinted with permission.)
Currently there is no reliable strategy to determine which arthroplasty patients will develop symptoms related to lower extremity thrombosis. Up to 50% of patients who developed thromboembolism after THA have no associated risk factors, underscoring the difficulty in identifying susceptible patients.8 Nonetheless, there are identifiable predisposing factors that have been associated with the development of symptomatic venous thromboembolism in this cohort of patients (Table 53-2). The most relevant risk factors that have been associated with the development of thromboembolic disease after hip surgery include a history of prior thromboembolic disease, obesity (body mass index >25), delay in ambulation after surgery, and female gender.7 Factor V Leiden mutation, antiphospholipid antibody syndrome, protein C and S deficiency, and impairment of the fibrinolytic system may increase the risk of symptomatic thromboembolic disease after hip arthroplasty in some patients. The presence of genetic polymorphisms, particularly prothrombin G20210A and AT III, has been associated with increased risk of thrombosis in patients undergoing THA.9 However, because the overall rate of thrombophilic disorders in the general population is low, preoperative genetic screening for THA patients has not been recommended.2,9–11
TABLE 53-2 CLINICAL RISK FACTORS ASSOCIATED WITH VENOUS THROMBOEMBOLIC DISEASE AFTER TOTAL HIP ARTHROPLASTY
| Risk Factors |
| Prior thromboembolic disease |
| Obesity |
| Female gender |
| Delayed ambulation after surgery |
| Advanced age |
| Paralysis |
| Malignancy |
| Cardiovascular disease |
| Fracture of the pelvis, hip, femur, or tibia |
| Hypercoagulable states |
| Antithrombin III deficiency |
| Protein C or S deficiency |
| Factor V Leiden deficiency |
| Antiphospholipid antibody syndrome |
| Dysfibrinogenemia |
From White RH, Henderson MC: Risk factors for venous thromboembolism after total hip and knee replacement surgery. Curr Opin Pulm Med 8:365-371, 2002; and Westrich GH, Weksler BB, Glueck CJ, et al: Correlation of thrombophilia and hypofibrinolysis with pulmonary embolism following total hip arthroplasty: An analysis of genetic factors. J Bone Joint Surg Am 84:2161-2167, 2002.
CHEMOPROPHYLACTIC REGIMENS
Various targets for anticoagulant drugs are outlined in Figure 53-2.
Warfarin
Warfarin remains the most commonly used agent in North America after THA.4 Warfarin exerts its anticoagulant effect by inhibiting the vitamin K–dependent carboxylation of clotting factors II, VII, IX, and X, as well as protein C and protein S. Warfarin has been demonstrated to decrease the prevalence of deep venous thrombosis in THA by approximately 60% and proximal venous thrombosis by 70% when compared with prevalence in patients who had not received prophylaxis.2 Warfarin’s primary advantages over other prophylactic options are its relatively low cost and its oral route of administration. However, there are several drawbacks related to its use. Warfarin has a delayed onset of action, which may render patients relatively unprotected in the early postoperative period, at which time they may be at greatest risk for the development of thrombosis. Moreover, frequent monitoring of either the prothrombin time or international normalized ratio (INR) is required for appropriate dose adjustment. In addition, warfarin interacts with other medications, herbs, and food products as a result of its metabolism in the cytochrome P450 system of the liver. Of note, the combination of warfarin and nonsteroidal anti-inflammatory agents has been shown to result in a 13-fold increase in hemorrhagic peptic ulcers in elderly patients.11a Finally, warfarin has been associated with a 1% to 5% incidence of major postoperative hemorrhage after hip arthroplasty.2,5
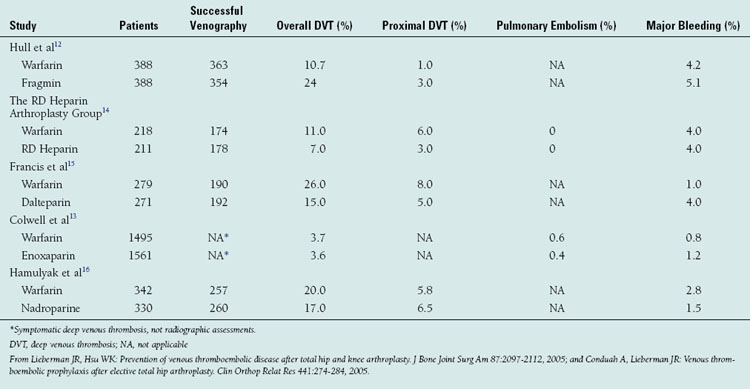
Warfarin’s efficacy has been compared with that of other prophylactic agents in both cohort studies and randomized clinical trials (Table 53-3).12–16 A recent meta-analysis was performed on all identified randomized controlled trials from 1966 to 1998, comparing low-molecular-weight heparins, warfarin, aspirin, low-dose heparin, and pneumatic compression devices.2,5 In this review, patients receiving warfarin had the lowest incidence of proximal deep venous thrombosis (6.3%) and symptomatic pulmonary embolism (0.16%). The risk of major postoperative bleeding in patients receiving warfarin therapy was no higher than that in patients treated with placebo. Warfarin has also been compared directly with low-molecular-weight heparins in a number of randomized multicenter trials.2,5 Collectively these studies have demonstrated a similar or lower incidence of asymptomatic deep venous thrombosis in patients receiving a low-molecular-weight heparin when compared with those receiving warfarin. However, in one study the rate of major bleeding episodes was found to be higher in patients receiving a low-molecular-weight heparin.2,4,5
TABLE 53-3 RESULTS FROM RANDOMIZED CLINICAL TRIALS COMPARING WARFARIN WITH LOW-MOLECULAR-WEIGHT HEPARIN AFTER TOTAL HIP ARTHROPLASTY
Warfarin prophylaxis is typically initiated the evening before surgery or the evening of surgery with a 5-mg, 7.5-mg, or 10-mg dose. Subsequent doses are based on the prothrombin time or INR. The target INR level is 2.0 for total joint arthroplasty patients.2 There are concerns that maintaining the INR at a higher level may result in an increase in bleeding events. With this approach, the target range for the INR is usually not attained until at least the third postoperative day. Some surgeons prefer to maintain the INR level below the true target level INR of 2.0 in order to limit the risk of bleeding. However, a lower INR may not provide optimal protection against thrombosis and is unlikely to eliminate the risk of bleeding.4 In a large cohort study, use of an adjusted nomogram was shown to provide effective and safe prophylaxis that was comparable to that provided by physician-adjusted dosing of warfarin in establishing and maintaining a therapeutic INR and limiting thrombus formation after total joint arthroplasty.17 Overall, warfarin is a safe and effective agent for thromboembolic prophylaxis after hip arthroplasty. However, it is more difficult to use because of the required monitoring and the multiple drug-drug interactions. Although warfarin may not be as effective as low-molecular-weight heparins in preventing venous thromboembolism, there is a lower risk of major bleeding complications.4
Unfractionated Heparin
Standard unfractionated heparin is a heterogeneous mixture of glycosaminoglycans with molecular weights ranging from 3000 to 50,000 daltons. Heparin acts via the binding affinity of a unique pentasaccharide with AT III. This interaction accelerates the inhibition of thrombin, factor IX, and factor Xa and is mediated by the simultaneous binding of both thrombin and AT III, which requires a minimum chain length of 18 saccharides to achieve ternary complex formation. Standard low-dose heparin (5000 units administered subcutaneously twice daily) is not currently recommended as prophylaxis after THA owing to its relatively low efficacy in preventing proximal thrombosis.2 Adjusted-dose heparin has been demonstrated to be more effective in limiting thrombus formation after arthroplasty.2 The anticoagulant effect of heparin is monitored by the activated partial thromboplastin time (aPTT). Although adjusted-dose heparin can provide effective prophylaxis after THA, daily monitoring of aPTT is cumbersome, and this prophylactic regimen is rarely used in current practice.
Low-Molecular-Weight Heparins
Low-molecular-weight heparins are a popular class of antithrombotic agents that do not require monitoring for an appropriate therapeutic effect to be achieved. These compounds are derived from unfractionated heparin via chemical or enzymatic depolymerization, generating fragments with molecular weights between 1000 and 10,000 daltons.18,19 Although low-molecular-weight heparins also enhance antithrombin activity, they differ from unfractionated heparin in their relative inhibition of thrombin and factor Xa. Heparin-mediated inactivation of factor Xa requires a unique pentasaccharide sequence that promotes the binding of antithrombin to factor Xa.19a
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree