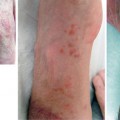
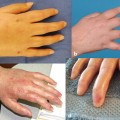
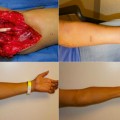
Fig. 6.1
Canine myocutaneous VCA model. a Myocutaneous flap harvested. b Flap inset and healed
Monkeys are not inbred, but the animals available for transplant research cannot be considered truly outbred either. Close attention to typing is mandatory if experiments are to be interpreted, particularly with the small numbers required in the modern research environment [39]. Common typing methods that must be employed to demonstrate genetic differences include mixed lymphocyte culture, serologic delineation of the class II loci (particularly the rhesus macaque DR region), one-dimensional isoelectric focusing (a biochemical characterization technique for both class I and class II), and polymerase chain reaction-based techniques for the highly polymorphic macaque exon 2 of MHC-DRB [39].
Canine Models in VCA
In our laboratory, we developed a preclinical canine model for VCA with a myocutaneous rectus abdominis allograft (Fig. 6.1) [42]. We then applied a clinically significant non-myeloablative hematopoietic stem cell transplant (HCT) regimen to induce tolerance to VCA allografts. This regimen was initially performed in DLA-identical littermate recipients and consisted of 200 cGy TBI before and mycophenolate mofetil (MMF)/ CSP given after HCT to control both GVHD and host-versus-graft (HVG) reactions. The regimen has been successfully translated into the clinic to treat human patients with both malignant and nonmalignant diseases by grafts from human leukocyte antigen (HLA)-matched related and unrelated donors without the need for testing in any additional animal models [43–45].
Using this regimen, we first demonstrated that donor-specific tolerance can be induced to a VCA after the establishment of mixed chimerism across a minor genetic barrier. All five animals transplanted with a vascularized myocutaneous tissue allograft demonstrated long-term acceptance of their transplant for greater than 1 year [33]. The allografts appeared normal with excellent hair growth with no evidence of rejection in the skin or muscle. In contrast, four control animals that were transplanted across the same barrier without immunosuppression rejected their allografts (15–30 days). The tolerant animals also demonstrated stable engraftment of their bone marrow transplants as demonstrated by the presence of donor granulocytes and lymphocytes.
We have also demonstrated that tolerance to the VCA is not dependent on the previous establishment of donor cell chimerism . Our clinically relevant model entailed the simultaneous marrow and VCA transplants using the same non-myeloablative HCT protocol. In this study, we observed 100 % acceptance of all components of the VCA, specifically skin and muscle, for all four dogs that underwent simultaneous transplantation of VCA and hematopoietic stem cells (HSC) for periods greater than 1 year. In addition, all of the tolerant dogs went on to accept a second non-vascularized skin graft while they rejected a third-party skin graft. These results confirmed that tolerance is not dependent on the previous establishment of stable mixed chimerism. In fact, tolerance may not be dependent on long-term engraftment of the donor bone marrow as evidenced by one of the four dogs that demonstrated initial engraftment of the HCT but had no detectable donor cells in the blood or bone marrow after week 12. Despite the loss of the HSC allograft, the VCA remained without any evidence of rejection. To control for the influence of the conditioning regimen, we also performed four transplants with the same non-myeloablative regimen but without any HSC infusion. All animals demonstrated acute rejection after the completion of the post-grafting immunosuppression. Currently, our focus has been modifying this regimen to use across greater genetic disparities and examining the need for long-term engraftment of donor cells for the maintenance of tolerance.
Swine Models in VCA
The swine model has been frequently used for the exploration and development of clinical protocols for the transplantation of VCA (Fig. 6.2). Ustuner et al. reported transplantation of a radial forelimb osteomyocutaneous flap between size-matched outbred swine with the use of a daily CSP, MMF, and prednisone oral regimen [46]. Of the eight swine, two sustained severe rejection, three demonstrated mild-to-moderate rejection, and three were free of rejection at the termination of the experiment at 90 days. No drug toxicity was evident in serum hematologic and chemical parameters of immunosuppressed animals. The Louisville group also examined the use of tacrolimus, MMF, and prednisone in the same swine model. Five of nine animals that survived to the study end at 90 days were noted to be free of rejection [47]. This work served as a basis for their human hand transplants performed under the same chronic immunosuppression regimen. However, this work did not attempt to induce tolerance to these allografts.
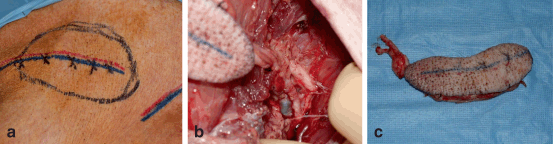
Fig. 6.2
Fasciocutaneous VCA swine model. a Flap design; outline, femoral and medial saphenous vessels marked. b Dissection of medial saphenous vessels to junction with femoral artery and vein. c Isolated fasciocutaneous VCA ready for transplantation. (Photographs courtesy of Dr. Curt Cetrulo)
The swine model has also served to explore techniques to induce tolerance to the VCA allograft. Initial work by Lee et al. sought to achieve host tolerance to musculoskeletal allografts through matching of the MHC antigens between donor and host swine with only a 12-day course of cyclosporine [48]. Allografts from MHC-mismatched donors treated with cyclosporine and allografts from MHC-matched (minor antigen mismatched) donors not treated with cyclosporine were rejected. However, allografts from MHC-matched donors treated with 12 days of cyclosporine showed no evidence of rejection until sacrifice up to 47 weeks after transplantation. This protocol did not induce tolerance to skin and when a cutaneous portion was added to the transplant, only one out of the six animals transplanted maintains tolerance to the entire allograft [28]. The majority demonstrated “split tolerance” where the skin was rejected but the muscle and bone survived long term. Attempts to extend this work utilizing high-dose tacrolimus rather than cyclosporine failed to induce tolerance to myocutaneous grafts across greater genetic disparities .
Hettiaratchy et al. sought to apply a mixed chimerism protocol to the same VCA model across greater genetic barriers (haploidentical and fully mismatched MHC) [49]. In these experiments, he used a nonmyeolablative protocol that combined anti-CD3 antibody, 150 cGY thymic radiation, and the infusion of donor hematopoietic cells with the VCA transplant [49]. These animals received either bone marrow or cytokine-mobilized peripheral blood mononuclear cells with 30 days of cyclosporine for post-grafting immunosuppression . As was noted in the previous experiments, split tolerance was again observed with rejection of the skin noted by day 60. In addition, all chimeric animals developed cutaneous GVHD approximately 70 days post transplant. While these cases did respond to treatment with immunosuppression, this complication limits clinical applicability.
HSC engraftment and stable mixed chimerism can be achieved in Massachusetts General Hospital (MGH) miniature swine conditioned with 100 cGY TBI and CD3-immunotoxin before transplant with 15 × 109 conditioned media-peripheral blood mononuclear cell (CM-PBMC) per kilogram and 45 days treatment with CSP A [42]. Two animals on this protocol received primarily vascularized skin flaps transplanted from the original donor on a sapheno-femoral vascular pedicle. Unfortunately, one animal died 46 days post transplant from unrelated complications, but another accepted this skin transplant indefinitely with follow-up of more than 1 year and no gross or histologic evidence of rejection at any time. This animal maintained stable, multilineage mixed chimerism, detectable in peripheral blood, thymus, and bone marrow and did not develop GVHD [43]. This was the first reported induction of skin tolerance in a large-animal model across an MHC barrier and provided proof-of-principle for the induction of tolerance of skin-bearing VCA using the mixed chimerism approach .
Recent work in the swine model from Kuo et al. demonstrated acceptance of a VCA using a combination of low-dose TBI (150 cGy), intrathymic irradiation, bone marrow infusion, and MSC transplant, followed by a short duration of post-grafting immunosuppression [50]. This protocol appeared to lead to prolonged acceptance of the allograft. The authors then modified the protocol to exclude administration of donor bone marrow, and again noted prolonged survival of the allograft [51]. This work suggested that the use of MSC alone may enhance survival of the transplant. However, neither donor chimerism nor donor-specific tolerance was addressed in their studies.
Nonhuman Primates
Trials to induce tolerance to VCA in nonhuman primates have been ineffective as well. There is no published report of a successful induction of tolerance to a VCA in the literature. In fact, until the advent of improved immunosuppression, even attempts to maintain the allograft with chronic immunosuppression failed. Daniel et al. and Stark et al. each published their experience with hand transplantation in a baboon model (Fig. 6.3) [52, 53]. They found thateven with the administration of high-dose CSP and steroids failed to prevent rejection. However, in a more recent publication, Gold et al. reported the ability to maintain a transplant for up to 65 days in select animals with CSP alone [54].
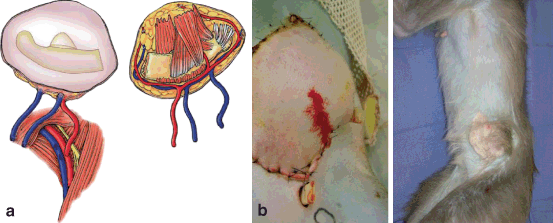
Fig. 6.3
Primate osteomyocutaneous VCA model. a Osteomyocutaneous flap design. b Flap inset and healed. (Photographs courtesy of Dr. Rolf Barth)
Barth et al. employed a similar mandibular allograft primate model and demonstrated long-term survival (177 days) with high-dose tacrolimus [55]. Unfortunately, in all of these monkeys, the VCA was inevitably rejected and the majority of the monkeys developed post-transplant proliferative disorder (PTLD). The addition of MMF to the same protocol allowed for the long-term survival of the allografts [56]. This study also evaluated the role of the inclusion of a vascularized bone marrow segment. Facial segments containing bone enjoyed prolonged survival until the immunosuppression was withdrawn, afterwards all allograft went on to reject. In contrast, transplants devoid of the mandibular component were all lost to acute rejection by day 15 [57]. The histologic evaluation of the five long-term surviving animals that later underwent immunosuppression withdrawal all demonstrated features consistent with chronic rejection, including neointimal proliferation and transplant vasculopathy [56]. The group also examined these animals for the presence of T-regulatory cells in the biopsy specimens and in the peripheral blood and found no correlation between presence of T-regulatory cells and the presence or the absence of rejection [58] .
There have been limited studies using co-stimulatory blockade in primate tolerance protocols. Barth et al. found that the addition of anti-CD28 to their protocol led to prolongation of the allograft but not tolerance [59]. In a recent unpublished experiment by Cendales et al. attempting to block both T cell co-stimulation with CTLA 4-Ig and antigen-presenting cells via LFA 3-Ig, it was unable to prevent rejection of a transplanted myocutaneous forearm flap.
Conclusions
Vascularized composite allotransplantation represents a paradigm shift in the reconstruction of complex facial defects and extremity loss. However, the application of VCA is currently limited by the need for chronic immunosuppression. The development for strategies to decrease or eliminate this requirement is key to the expansion of this technique. Initial studies in small-animal models allow for the cost-effective exploration of multiple variables in the development of tolerance protocols. However, the translation of protocols developed in small-animal models often fails when applied to large-animal models. The use of large-animal models is critical for the further development and advancement of clinical VCA transplantation.
References
1.
Breidenbach WC 3rd. Update on hand transplantation: Louisville. In: American Society of Reconstructive Transplantation: 2010. Chicago; 2010.
2.
Lantieri L. Face transplant: a paradigm change in facial reconstruction. J Craniofac Surg. 2012;23(1):250–53.PubMed