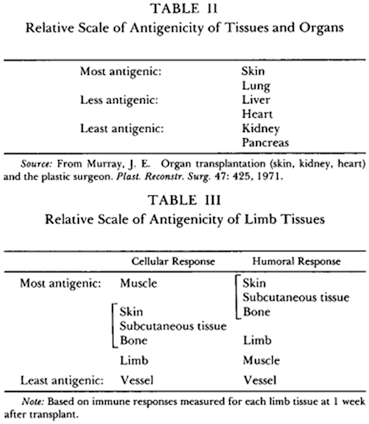
The differential immune response generated by different organs/tissues from the same donor results in acceptance of one and rejection of another. This phenomenon of “split tolerance” has been extensively studied in the laboratory.
Reasons for High Immunogenicity of Skin
Skin is the largest organ of the body and is the first point of contact with the external environment. This has resulted in a specialized and potent immunological apparatus in the skin and surrounding tissues. Structurally, it consists of the epidermis and dermis. The epidermis is chiefly composed of ectodermal-derived keratinocytes and a smaller proportion of Langerhans cells , Merkle cells, and melanocytes. The dermis supports the epidermis and is composed of fibrous connective tissue (collagen and elastin) in a matrix of ground substance. Based on the difference in connective tissue density and arrangement, the dermis is divided into two layers: the superficial papillary dermis and the deeper reticular dermis which overlies the subcutaneous fat. Other important components of skin include the hair follicles, sebaceous, ecrine, and apocrine glands. The cutaneous vasculature is composed of two plexuses: the superficial and a deep plexus of arterioles and venules. Paralleling the blood supply of the skin is a lymphatic system that serves to allow Langerhans’ cells to travel the regional lymph nodes.
The mechanisms of cutaneous defense consist of both innate and adaptive immune responses . The innate immunity consists of a physical barrier of stratum corneum and its various antimicrobial products including secreted lipids. In addition, antibacterial antibodies (immunoglobulin (Ig) A) are secreted by the sweat and sebaceous glands.
The adaptive cutaneous immunity is orchestrated through the “skin associated lymphoid tissue” (SALT) analogous to the gut-associated lymphoid tissue (GALT). This includes the Langerhans’ cells, resident and migratory lymphocytes, keratinocytes, etc. The skin is rich in dendritic cells (DCs) which are the predominant antigen-presenting cells in the body. These Langerhans cells are abundant in the epidermal and dermal tissues and play a key role in the initiation of immune responses in the skin. They constitutively express major histocompatibility complex (MHC) class I antigens. Upon stimulation, the DCs and skin keratinocytes present class II antigens, intercellular adhesion molecule 1 (ICAM-1) and pro-inflammatory cytokines . The chief function of these cells is to process and present antigens encountered in the epidermis to naive T cells and thus initiate the adaptive immune response. Circulating skin-homing lymphocytes are a part of the cutaneous immune response and normal human skin demonstrates extravascular T lymphocytes—both CD4 and CD8. This is relevant to interpretation of skin biopsies in the transplant situation.
In the context of VCA transplantation, the cutaneous Langerhans’ cells, which constitute 2–4 % of epidermal cells, are thought to play a crucial role in the priming of naive host T cells against the donor antigens. This results in the rejection response. Keratinocytes, which compose 90 % of epidermal cells, support the cell-mediated immune response. The DCs present in the dermis (dermal DCs) are able to migrate to the secondary lymphoid tissues. This property enables them to carry skin antigens to the host’s lymphoid structures leading to initiation of the rejection phenomenon.
In addition, skin possesses tissue specific soluble antigens that are thought to play an important role in its antigenicity.
Immune Activation
Following transplantation, skin DCs migrate from the graft via the lymphatic vessels to the recipient’s lymph nodes. They may present donor antigens to the recipient in two ways: the direct path where the host T cells recognize the donor MHC molecules present on the donor DCs and the indirect path where the donor peptides are bound to recipient DCs and then present them to the recipient T cells. Allo-recognition by either mechanism can trigger the rejection of the allogeneic skin graft. In addition, natural killer (NK) cells may be activated by the absence of self MHC class I molecules on allogeneic cells—this leads to direct killing of donor cells and the production of pro-inflammatory cytokines such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α [10].
Role of Adhesion Molecules
The infiltration of alloantigen specific T cells into the skin of VCA grafts has been considered the key feature of acute rejection in composite tissue transplants . Adhesion molecules are vital for the function of immune cells. These molecules help activate leukocytes and convert them from an inactive, non-sticky state to an adhesive state [11]. This permits the activated cells to adhere to the vascular endothelium and thus migrate to inflamed tissues. The expression patterns of adhesion molecules are specific for each population of cells [12].
Various adhesion molecule types have been identified and broadly belong to two groups: selectins and integrins. It is currently thought that selectins (E, P, and L type) are important in leukocyte infiltration of skin in inflammation . Leukocytes also express integrins (lymphocyte function-associated antigen (LFA)-1 and macrophage-1 antigen (Mac-1)) which bind to ICAMs (ICAM-1 and 2) expressed on vascular endothelium.
Following transplantation, there is a unique injury related to restoration of blood flow following a period of ischemia: ischemia–reperfusion injury. This results in upregulation of adhesion molecules and generation of damage-associated molecular patterns (DAMPs). This leads to recruitment of lymphocytes [13] with potential adverse effect on graft—both acute and long-term injury [14]. Blocking adhesion molecules has been studied in experimental models to blunt the effect of ischemia–reperfusion injury . P-selectin blockade has been shown to reduce ischemia–reperfusion injury following liver transplantation in mouse models [15] and in a phase II clinical study [16]. In the study by Busuttil [16], the selectin antagonist known as recombinant P-selectin glycoprotein ligand IgG (rPSGL-Ig) was shown to significantly reduce the incidence of poor early graft function in liver transplant recipients, including those with a high donor risk index.
Adhesion Molecules in VCA
Study of skin biopsies from hand transplant recipients has demonstrated a strong correlation of LFA-1, ICAM-1, and E-selectin with the severity of acute rejection [17]. This finding was followed up with an elegant series of experimental studies by the Innsbruck group [17]. In a rat hind limb VCA model, Effomycine M was used subcutaneously to inhibit E and P-selectins. Long-term graft survival was demonstrated in five of six animals. Use of other agents to block ICAM-1 and LFA-1 was also reported to prolong graft survival significantly [11]. The ability to use these agents locally in the case of VCA transplants provides opportunities that are not feasible in solid organ transplantation. If this path can be further explored, it has the potential to target the early phase of immune activation with minimal systemic effects and thus facilitate lower systemic immunosuppression.
Rejection in VCA
By 2006, three different classification systems to grade acute rejection in VCA transplants had been published [18–20]. As the clinical volumes began to grow, there was a need to develop consensus on the issue . This was addressed at the Ninth Banff Conference on Allograft Pathology in 2007. The consensus group on composite tissue allotransplantation published its recommendation in 2008 [21]. Guidelines included the following: (a) specimen size—one “4-mm” punch biopsy of skin taken from the most red and indurated area of involved skin, (b) sample must have epidermis and adnexa, dermis, subcutaneous tissue, and vessels, and (c) slide preparation with hematoxylin–eosin (H&E) and periodic acid–Schiff (PAS) stains.
Clinical findings of rejection include mild pink discoloration, gradual erythema, macules progressing to red infiltrated lichenoid papules with or without limb edema, and onychomadesis in advance rejection [21]. Skin lesions can be either scattered over the allograft or present in a confluent pattern. In the Banff 2007 working classification, the area of graft involvement was graded as follows: < 10 %, 10–50 %, and > 50 % of the graft. However, this description has not been widely used in subsequent literature as this is not vital to grading the rejection event. The microscopic appearance is the basis for grading and is shown in Table 7.1.
Table 7.1
The Banff2007 working classification of skin-containing composite tissue allograft pathology [21]
Grade 0 | No or rare inflammatory infiltrates |
Grade I | Mild. Mild perivascular infiltration. No involvement of the overlying epidermis |
Grade II | Moderate. Moderate-to-severe perivascular inflammation with or without mild epidermal and/or adnexal involvement (limited to spongiosis and exocytosis). No epidermal dyskeratosis or apoptosis |
Grade III | Severe. Dense inflammation and epidermal involvement with epithelial apoptosis, dyskeratosis, and/or keratinolysis |
Grade IV | Necrotizing acute rejection. Frank necrosis of epidermis or other skin structures |
Grade 1 rejection (mild) includes mainly lymphocytic perivascular aggregates in the dermis without epidermal involvement. With progression, the cellular infiltrate spreads into the epidermis. In severe cases, there is dense involvement of epidermis with apoptosis, dyskeratosis, and keratinolysis which could end up in frank necrosis of skin (Table 7.1 [21]).
While acute rejection in hand transplantation typically presents with maculopapular erythematous rash that is diffuse or patchy/focal over the forearms and dorsum of hands, a variant form has been described to occur rarely [22]. This atypical form involves the palmar skin and nails and has been attributed to repetitive mechanical stress of the palm. The features include red papules and lichenification of the palmar skin and dystrophy of the nail. The response to steroids was poor and resolution required the use of lymphocyte depleting agents Thymoglobulin and alemtuzumab .
Immunohistochemical Studies of Rejection in VCA
Cendales [23] studied the cellular infiltrate seen during acute rejection using immunohistochemical staining. The study of 29 specimens from both hand and abdominal wall transplants showed that the cells were predominantly CD4 + in milder cases and CD8 + in advanced cases. Hautz [17], in a more recent study, described a different pattern of infiltrate. In a study of 174 skin biopsy specimens collected over a 9-year time scale from five hand transplant recipients, the author showed that the perivascular infiltrate was predominantly CD3 + T lymphocytes—a tendency for a predominance of CD8 positive lymphocytes in milder cases and CD4 positive cells in advanced cases was noted. During rejection, 10–50 % of cells were identified to be CD68 + histiocytes/ macrophages. The numbers were increased during higher grades of acute rejection. CD 20 + B cells were rarely detected (0–5 %) in the skin of hand transplant recipients. In addition, Fox p3 and indoleamine 2, 3-dioxygenase expression correlated with the severity of rejection—suggesting a tendency toward self-limitation of the alloimmune response during the rejection process in VCA [24].
Humoral Immunity in VCA
The role of HLA antibodies in solid organ transplantation is well established. Since the landmark studies of Patel and Terasaki [25, 26], pre-transplant identification of donor-directed human leukocyte antigen (HLA) antibodies (donor-directed HLA-specific alloantibodies (DSAs)) has been a critical prelude to renal allotransplantation [27]. The presence of DSAs is largely a contraindication in renal transplantation. Recent innovations such as the use of desensitization techniques [28] have enabled successful transplantation in highly sensitized individuals. The development of DSAs following transplantation has been associated with episodes of acute rejection, chronic rejection, and graft loss in renal transplantation [29, 30].
The ability to detect DSAs has improved significantly in the past few years with the development of solid-phase detection assays [27]. This enhanced sensitivity and specificity in DSA detection has led to many unanswered questions regarding their relevance [31].
The role of preformed HLA antibodies in VCA transplantation has not been studied in depth. Although most VCA transplant centers use pre-transplant crossmatch testing [32, 33], the precise role DSAs play in hand or face transplantation is yet to be determined. A recent study [34] in Wistar Furth (WF) rats demonstrated that VCA grafts are rejected in an accelerated but not hyperacute fashion in the presence of allosensitization and preformed DSA. Additionally, this rejection was mainly cell-mediated and differed mechanistically from renal transplantation.
DSAs have developed during the follow-up of hand transplant recipients (de novo DSA) but have not been clearly shown to have adverse effects [33, 35]. C4d is a by-product of complement activation and the presence of staining for C4d on histopathology is considered a hallmark of acute antibody-associated injury of the allograft [36]. C4d deposition has been investigated in skin biopsies from VCA recipients with mixed results. Kanitakis [37] reported absence of C4d deposition in a study of 60 biopsy specimens obtained from four VCA recipients (three hand and one face). However, Landin [38] reported the occurrence of C4d deposits in the capillaries of skin biopsy specimens from two hand transplant recipients—both during and in the absence of clinical rejection episodes. Thus, further studies are needed with long-term follow-up of VCA recipients to further assess the role of preexisting and de novo DSAs in the field of VCA.
Regulatory T Cells
Regulatory T cells are thought to counter rejection and promote tolerance in the setting of transplantation. Many types of regulatory T cells have been identified in the recent past. These include CD8 + T cells, CD4-CD8- double negative T cells, CD8 + CD28-, natural killer (NK) T cells, and γδ T cells [39–42]. But the best studied are the CD4 + regulatory T cells (Tregs). These have been characterized by high and stable expression of surface interleukin (IL)-2 receptor α chain (IL-2Rα, CD25hi) and the transcription factor, fork-head box protein 3 (FoxP3) [43]. These cells are derived from thymus and are CD4 + CD25 + FoxP3 + and are referred to as natural Tregs (nTregs), in contrast to the induced Tregs (iTregs) which are generated in the periphery and whose activation requires T cell receptor engagement and cytokines [44]. Tregs have been shown to prevent rejection of allogenic skin grafts in T cell deficient nude mice given CD25− T cells [43]. In a murine skin transplant model following thymectomy and partial T cell depletion, in vitro expanded Tregs have been shown to induce donor-specific transplantation tolerance [45]. Trials are currently in the pipeline to use adoptive Treg cellular therapy in inducing transplantation tolerance [44].
Studies in human hand transplantation have demonstrated the presence of Tregs in transplanted skin. Intracellular staining of skin biopsy with highly specific monoclonal antibodies (mAbs) and measuring the FoxP3 messenger RNA (mRNA) expression has demonstrated the presence of FoxP3 positive cells in the grafted hand. In addition, these cells showed immunosuppressive properties when isolated in culture. These cells were found to be present as far out as 6 years posttransplantation [46]. It has been suggested that the presence of these cells could play a role in the long-term survival of VCA grafts [46].
Vascularized Bone Marrow Transplant
In the experimental setting, VCA grafts (face and limb) are thought to function as a vascularized bone marrow transplant (VBMT). Hewitt [47] established macrochimerism with vascularized limb allografts from Lewis X Brown-Norway F1 to Lewis rats and reported long-term survival in eight recipients treated with cyclosporine. When immunosuppression was discontinued, two of eight animals did not show histologic evidence of acute rejection.
Based on laboratory data, it was widely anticipated that clinical hand transplantation would induce chimerism due to the viable bone marrow component of the graft. However, this expectation has not been fulfilled. Peripheral blood kinetic studies on two hand transplant recipients failed to demonstrate either donor macrochimerism or donor-specific hyporesponsiveness in mixed lymphocyte reaction [48




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree