Hand fractures account for about 1.5% of all emergency room visits and 40% of upper extremity fractures. Unfortunately, complications occur following these injuries. When the underlying osseous structure is affected with nonunion or malunion, it can further compromise hand function, therefore diagnosis and treatment of these complications is an important part of caring for patients with hand fractures. The decision to intervene must be based on the likelihood of achieving the desired correction, and improving the function, of the hand. This article reviews principles of diagnosis and treatment of nonunions and malunions, including conditions affecting the thumb and pediatric patients.
Fractures in the upper extremity are common, accounting for about 1.5% of emergency department visits, with hand fractures comprising 40% of all upper extremity fractures. Complications can and do occur, making their diagnosis and treatment an important part of caring for patients with these injuries.
Malunions are common in the metacarpals and phalanges, although not all are clinically significant. The common fifth metacarpal neck, or boxer’s fracture, usually heals with an apex dorsal deformity, but this is rarely problematic. By contrast, those with rotational or angular deformities often affect hand function, and treatment is necessary. Nonunions, on the other hand, are uncommon, but when they occur are often significant and inevitably require treatment. Nonunions are often associated with other conditions such as tendon and nerve injuries, and as a result sometimes salvage procedures, such as arthrodesis or amputation, are the best treatment.
Nonunion
Delayed unions often occur in the hand, but will eventually heal and do not require intervention, whereas nonunions are more common with infection and open fractures, and these will inevitably require operative treatment. Occasionally they may result from metabolic abnormalities, such as low calcium or vitamin D, but once the metabolic abnormality is corrected, union will typically follow. Radiographic appearance alone is not a reliable indicator of nonunion, but with clinical instability or deformity, treatment should be considered because prolonged immobilization is poorly tolerated and permanent stiffness will occur.
Timing for the diagnosis of nonunion is variable, but generally requires consecutive radiographs without signs of progressive healing for a period of 4 to 6 months. The pathophysiology of the nonunion is hypertrophic or atrophic, with atrophic being much more common in the hand. Hypertrophic nonunions display callous formation, but without bridging callous between fracture segments. With rigid fixation, union will typically occur. Atrophic nonunions do not exhibit callous and often reveal resorption at the site of the fracture. Resorption may be secondary to impaired blood supply, infection, metabolic conditions such as smoking or diabetes, or soft-tissue interposition between the fracture ends. Technical difficulties from a previous operative procedure, such as overdistraction of the fracture site, devascularization from exposure, or inadequate fixation can also lead to nonunion. Preoperative blood work, including a white blood cell count, erythrocyte sedimentation rate, and C-reactive protein, can be helpful and provide a means to follow recovery in the event of an infection. Intraoperative bone cultures should be obtained to determine specific organisms and to guide antibiotic choice. Surgical principles include eradication of any residual infection, debridement of nonviable bone, and stabilization of the fracture segments. Bone graft is often required to achieve union.
Arthrodesis is useful for articular nonunions associated with joint stiffness. This digit is likely to remain stiff following healing of the nonunion, and arthrodesis allows for internal fixation to cross the joint, allowing for a longer implant and more stability. Both the joint and nonunion site should be debrided, bone grafted, and stabilized ( Fig. 1 ).

Nonunions commonly occur in the tuft of the distal phalanx, but are rarely symptomatic. These fractures are typically the result of crush injuries and are associated with nail bed injuries. Despite nail bed repair some of these will fail to unite, which may result in an unstable tip of the finger and, depending on the digit, may be problematic for the patient. When a significant amount of bone is missing, amputation revision may be the best treatment, as bone grafting on the tip of the phalanx is prone to resorb.
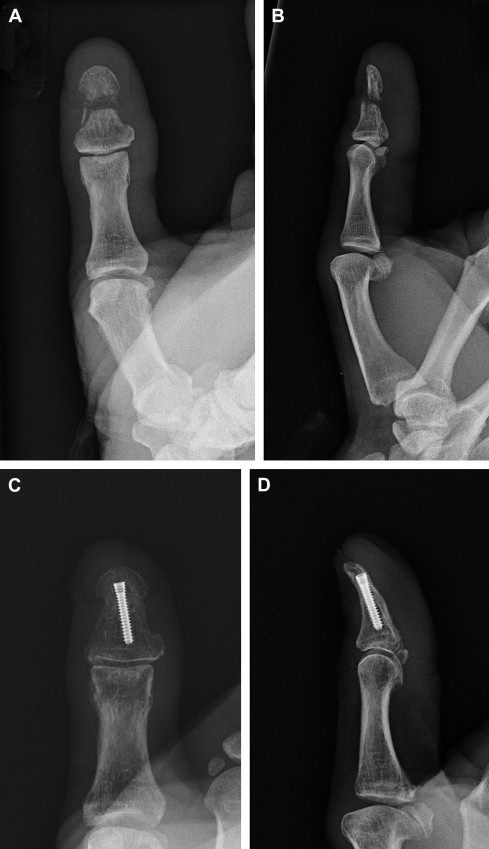
When the nonunion is in the shaft of the distal phalanx, this can often be treated with compression across the nonunion site, either through an open approach or with percutaneous compression screw placement ( Fig. 2 ).
Metacarpal, Proximal, and Middle Phalanges
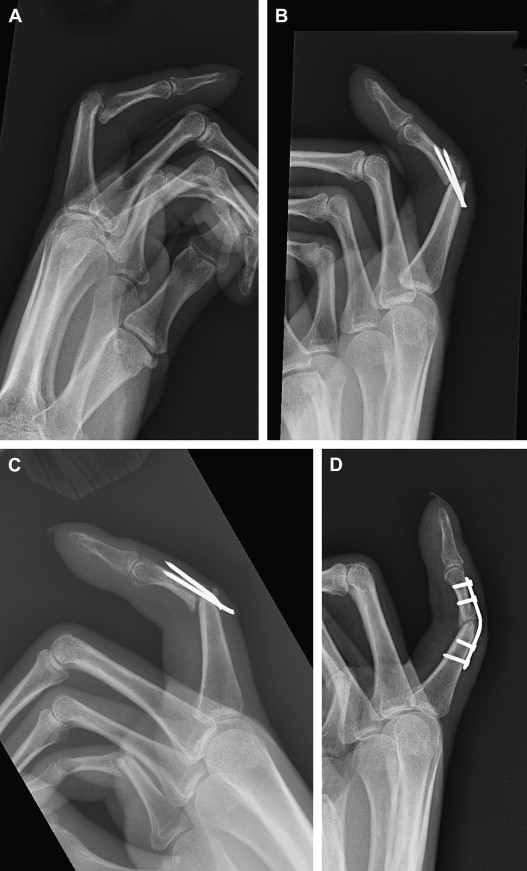
For shaft nonunions, rigid fixation is preferred when there is adequate soft-tissue coverage. The plate size is typically larger than would be used for an acute fracture in the same location. Unfortunately, especially in the phalanges, the soft tissue is often compromised, making plate fixation impossible ( Fig. 3 ). The nonunion site is exposed and nonviable bone is debrided. The void is filled with bone graft and fixation is applied. Early motion is preferred to promote tendon gliding and to minimize adhesions, as these digits are typically stiff from the previous injury.
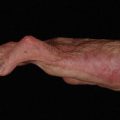
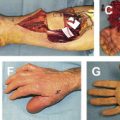
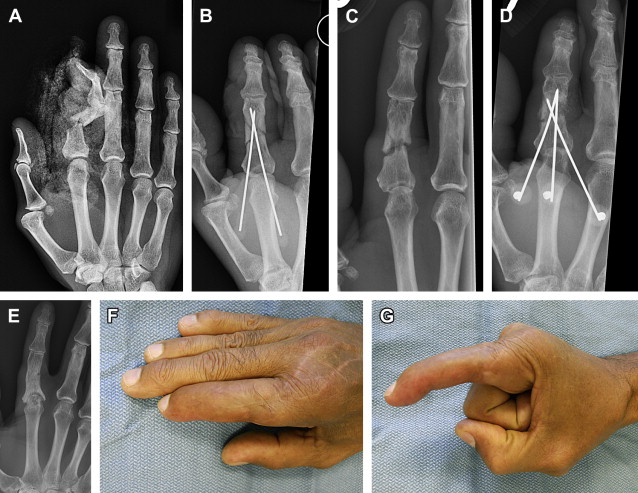
Amputation should be considered for cases with sensory loss, stiffness, persistent infection, or poor soft-tissue coverage. A stiff digit is often a liability to hand function and, even if the nonunion were to heal, the digit remains stiff and can impair hand function ( Fig. 4 ). Recovery following amputation is often rapid, and leads to improvement in function of the hand.
Nonsurgical treatment of nonunions with bone stimulators has been described. An external bone stimulator is applied over the skin or cast, and ultrasonic or pulsed electromagnetic waves are used to induce healing. Current evidence for the effectiveness of external bone stimulation in the hand is limited to case reports, therefore further evidence is needed to determine the true efficacy of this treatment.
Malunion
Malunions occur when a fracture heals in nonanatomic position; this can result in rotation, angulation, or shortening to varying degrees. Rotational deformities manifest with scissoring or crossing of the fingers in flexion ( Fig. 5 ). This appearance is often not evident with the fingers in extension, but can result in weakness and difficulty with dexterity. These rotational deformities typically occur following oblique or spiral fractures. Lateral angular deformities result from either intra-articular malunion with displacement of one condyle or with comminution and collapse on one side. These deformities are often evident with the digits in extension and are exacerbated with flexion, resulting in scissoring of the digit. When the fracture is inadequately treated, these deformities may result in a malunion.
Metacarpal fractures and malunions typically have an apex dorsal angulation secondary to the pull of the intrinsic tendons. Proximal phalanx fractures and malunions typically have apex volar angulation, whereas fractures of the middle phalanx have variable angulation depending on their location in relation to the flexor digitorum superficialis (FDS) insertion, with those proximal to the FDS insertion typically apex dorsal and those distal being apex volar.
Shortening of the digit typically occurs following healing of a comminuted fracture or those with segmental bone loss. Angular deformity (dorsal-volar direction) can create relative shortening (without actual bone loss), affecting the extensor mechanism. Strauch and colleagues demonstrated that 2 mm of metacarpal shortening results in 7° extensor lag, but this may not be as relevant because of the ability of the metacarpophalangeal (MCP) joint to hyperextend. Proximal phalanx malunions with apex volar angulation may result in pseudoclawing, with hyperextension of the MCP and extension lag at the proximal interphalangeal (PIP) joint. Vahey and colleagues reported that 1 mm of shortening results in 12° of lag when the shortening is in the proximal phalanx, with increasing angulation resulting in increased lag.
Malunions Involving the Distal Interphalangeal Joint
Malunions of the distal phalanx articular surface are common as mallet fractures heal, many times as fibrous union, but these tend to remodel. Long-term osteoarthritis is possible radiographically, but there is no evidence to determine the incidence of clinical symptoms. When an extension lag is present at the distal interphalangeal (DIP) joint and volar plate laxity allows hyperextension at the PIP, a swan-neck deformity results that can adversely affect hand function. Treatment is directed at restoring the articular surface and thus minimizing the extension lag at the DIP joint, which will correct the secondary PIP joint hyperextension ( Fig. 6 ).
Malunions Around the Proximal Interphalangeal Joint
When the fracture is in or near the joint and heals in a malunited position, deformity will result. Such deformity can include stiffness, pain, and rotational or angular deformities, affecting the function of the entire hand. Depending on the presence of arthritis, treatment may be with corrective osteotomy, arthrodesis, or arthroplasty, with autogenous tissue or a prosthetic implant. Arthrodesis and prosthetic arthroplasty are typically reserved for patients who have developed arthritis. Arthrodesis is preferred in the index finger because of the lateral stress from pinch, whereas arthroplasty is generally preferred in the other fingers to preserve motion and grip strength. Arthroplasty can be expected to relieve pain, but postoperative motion is similar to preoperative motion.
Intra-articular malunions at the base of the middle phalanx are often the result of dorsal fracture dislocations, whereby the contour of the middle phalanx is lost and resultant dorsal subluxation of the middle phalanx is present. When addressed early, before the onset of arthritis, this can be treated by resurfacing the base of the middle phalanx. The most common forms are volar plate arthroplasty hemi-hamate arthroplasty.
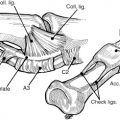
The indications are the same for both procedures and the decision is based on the surgeon’s preference, but the current trend seems to be for the hemi-hamate arthroplasty, based on the greater number of articles in the recent literature. Both procedures are performed through the volar approach using a Brunner (zig-zag) incision. The digital neurovascular bundles are mobilized to prevent injury when the joint is exposed. The flexor sheath is opened between the A2 and A4 pulleys, and the flexor tendons are retracted to expose the volar aspect of the PIP joint. The volar plate is released distally and the collateral ligaments are released from the middle phalanx. The joint is exposed by retracting the neurovascular bundles and flexor tendons, and hyperextending the digit (“shotgunning”), allowing the dorsal aspect of the proximal and middle phalanges to come together and thus allow visualization of the entire articular surface ( Fig. 7 ).
For volar plate arthroplasty, it is important to excise the collateral ligaments. A transverse mark is made in the middle phalanx, and the volar base is removed to create a trough where the volar plate can be advanced. The bone is cut, and the remaining bone is debrided to create a smooth surface into which the volar plate can be advanced. The joint is repositioned, and the volar plate is advanced and secured with transosseous sutures and tied over the dorsal aspect of the middle phalanx. The joint is pinned in slight flexion for 3 weeks and then motion is begun with an extension-blocking splint.
The hemi-hamate arthroplasty is performed through the same exposure, shotgunning the joint open after release of the collateral ligaments (not excised, as they are used during closure to provide additional lateral stability) and retraction of the flexor tendons. A transverse mark is made in the middle phalanx and bone is removed, creating a ledge on which to place the hamate graft. Dimensions of the graft are measured in dorsal-volar, radial-ulnar, and proximal-distal dimensions. Following preparation of the middle phalanx, the hand is pronated and the carpometacarpal joint of the fourth and fifth finger is confirmed with fluoroscopy. Following exposure of the dorsal hamate through a dorsal transverse incision, the dimensions of the defect are transferred to the hamate and the graft is harvested ( Fig. 8 ).
It is preferable to harvest a graft slightly larger than anticipated, and trim it to fit the middle phalanx. A portion of the proximal dorsal hamate can be removed to allow proper contour as the graft is harvested. The graft is secured with small screws or headless compression screws, providing stable fixation and allowing for early motion. The thickness of the cartilage on the hamate is often thicker than the base of the middle phalanx, giving the radiographic appearance of malpositioned graft, but this can be ignored as the positioning is confirmed visually during placement of the graft and confirmed following fixation. Care must be taken when placing the graft to recreate the curvature of the base of the middle phalanx. If the graft is placed too vertical, the middle phalanx will sublux in a dorsal direction—stability for this joint is based on the recreation of the contour of the base of the middle phalanx ( Fig. 9 ). Postoperatively, motion is begun around the fifth day with a figure-of-8 splint to provide lateral stability and to block the final 20° of extension. Biomechanical cadaver studies have demonstrated this graft to be suitable, with minimal donor site morbidity.