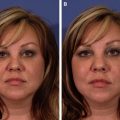
Disorders of hyperpigmentation encompass a plethora of pigmentary problems that can range from inherited to acquired. This article focuses on two prevalent disorders of hyperpigmentation and their treatment: melasma and postinflammatory hyperpigmentation. Each represents an acquired disorder of dyspigmentation with multifactorial etiology, which preferentially affects darker phototypes. Treatment can require a combination of medical, surgical, and laser modalities, as well as patience by both physician and patient. Treatment is limited mainly by the skin phototype of the patient, as darker skin types are more susceptible to adverse effects of treatment.
Disorders of hyperpigmentation are as common as they as are distressing. The color of the skin is the cumulative addition of not only the amount but also the distribution of melanin within the epidermis and dermis. The color of the skin that is portrayed is the result of melanin’s light absorption and subsequent reflection. Therefore, disorders of hyperpigmentation are the result of an increase in melanin production and even a change in density of activated melanocytes. In addition, the skin can become discolored as a result of deposition of medications as well as elements such as heavy metals. Labile melanocyte responses to injury or inflammation in skin of color can result in an increased prevalence of pigmentary disorders. Hyperpigmentation can be diffuse, circumscribed, linear, or reticulated, and such patterns can aid in a specific diagnosis. Two prevalent disorders of hyperpigmentation are melasma and postinflammatory hyperpigmentation (PIH). These disorders can be very concerning for patients and therefore treatment is greatly sought after. The treatment of hyperpigmentation is multifactorial, and can require multiple modalities as well as time and patience. These disorders also affect skin-of-color patients preferentially and therefore there is an added component of concern when treating skin of color patients, as one does not wish to depigment the skin in the treatment process. Both melasma and PIH can be very problematic and distressing for patients. The treatment of both can pose a challenge for the physician. Both disorders are discussed in this article, with a focus on a multimodality approach to treating pigmentary disorders. Often one treatment option is not enough, and a multifocus approach needs to be used.
Disorders of hyperpigmentation
Melasma
Melasma, also called chloasma or the mask of pregnancy, is a common disorder of hyperpigmentation that preferentially affects women. It is a circumscribed hypermelanosis with characteristic symmetric hyperpigmented patches occurring most frequently on the face, but can occur on the extensor arms. Melasma develops and progresses slowly and is often associated with hormonal changes, underlying genetic factors, and exposure to ultraviolet (UV) light as well as heat. In the United States melasma affects about 5 to 6 million individuals, and in one study the incidence of melasma in males was about 5% to 10%. It is more common among the Hispanic, Asian, African, and Middle Eastern populations, and tends to persist longer in those of darker phototypes. Melasma is an extremely prevalent and concerning problem in the Latino population. Sanchez and colleagues reported that melasma constitutes 8.2% of the diagnosis encountered in a Latino private practice population. Known exacerbating factors include pregnancy, oral contraceptives, and sun exposure. The pathogenesis, while not completely elucidated, is thought to involve UV exposure, or another exacerbating factor, in conjunction with hyperfunctional melanocytes that produce increased amounts of melanin. UV irradiation is thought to play the central role, and this is supported by the observation that melasma tends to improve during the winter months and by involvement of sun-exposed areas. Documented exacerbating factors are hormonal estrogen and possibly progesterone, medications such as phenytoin-related anticonvulsants and phototoxic medications, as well as increased expression of c-kit and stem cell factor within lesion skin. Perez and colleagues reported that fertile women who developed melasma without ever having been pregnant or on oral contraceptive medications may show a mild ovarian dysfunction consistent with polycystic ovarian syndrome. The melanocytes of melasma-affected skin have been shown to be highly dendritic, exhibit rapid DNA synthesis on UV sun exposure, and multiply rapidly. On histologic examination melanin deposition is seen in all layers of the epidermis, as well as an increased number of dermal melanophages.
In clinical terms facial melasma is divided into 3 patterns: 1) Centrofacial; 2) Malar; 3) Mandible. The centrofacial area is the most commonly affected area, seen in about two-thirds of patients. The malar area is the second most common, occurring in about 20% of patients, followed by the mandible area in about 16% of patients. Melasma is also subclassified into 4 subtypes based on illumination by Wood’s lamp: epidermal, dermal, epidermal and dermal (mixed), or intermediate. Lesions composed of epidermal pigment deposition are said to accentuate on Wood’s lamp illumination and those that are composed mainly of dermal pigmentation become less conspicuous or blend in on Wood’s lamp illumination. Melasma can be very disturbing for patients, and frustration can set in on recalcitrance to treatment. Factors that contribute to the severity of melasma are the surface area affected, intensity of pigmentation relative to the surrounding skim, and homogeneity of the lesions, with more surface area, 3 or more shade differences, and more homogeneous lesions all considered to be more severe.
Postinflammatory Hypermelanosis (PIH)
PIH, or postinflammatory hypermelanosis, is another frequently encountered, cosmetically concerning disorder of hyperpigmentation. As the name implies, PIH is an acquired hyperpigmentation that involves areas of prior cutaneous inflammation, allergic contact, irritant reactions, or trauma such as burns and friction. It can also occur after medication reactions or at sites of vesiculobullous diseases. Of course, cosmetic procedures such as chemical peeling, cryosurgery, laser therapy, intense pulse light therapy, and fillers can all produce PIH and therefore patients should be informed about the risk of such a development. PIH can affect all skin phototypes and is prevalent among the skin-of-color population. PIH can occur anywhere on the skin surface including the mucous membranes, and becomes apparent in the areas of inflammation once the initial erythema resolves. Patients of any age can be affected, and the incidence is equal in men and women. Although it occurs in all skin types, PIH may be more apparent in phototypes III to VI. Moreover, in these skin types the hypermelanosis may last longer and sometimes never fades completely. Halder and colleagues reported in 1983 that pigmentary disorders, other than vitiligo, were the third most common dermatoses among African American patients but were the seventh most common dermatoses among Caucasian patients. In 2007 Alexis and colleagues confirmed this observation by reporting that dyschromias was the second most common diagnosis among African American patients, whereas dyschromias did not make the top 10 most common diagnoses among Caucasian patients.
The pathogenesis of PIH depends on where the pigment resides. In the epidermal form there is an increase in melanin production and dendritic transfer to keratinocytes. In mice and possibly humans, mediators of inflammation such as prostaglandins E2 and D2 may enhance pigment production. In dermal hypermelanosis, melanin enters or “drops” into the dermis via a damaged epidermal basement membrane secondarily to the inflammatory process. This pigment incontinence is phagocytosed by the dermal melanophages where it resides. Some investigators report that patients with skin of color are more apt to develop postinflammatory pigmentation because of the large amount of melanin contained with the melanosomes within the epidermis. Others believe that the amount of PIH is related more to the individual’s type of melanocyte categorized as normal, weak, or strong. The difference is that weak melanocytes, after an inflammatory insult, lead to a decreased production of melanin, giving rise to clinical hypopigmentation, whereas strong melanocytes produce increased amounts of melanin after an inflammatory response, resulting in hyperpigmentation. Normal melanocytes remain unaltered, producing appropriate quantities of melanin. Although melanin is increased in this disorder, the number of melanocytes remains the same. On dermatopathologic examination, the epidermal form of PIH shows increased pigment in epidermal keratinocytes whereas the dermal form is characterized by melanin deposition within dermal macrophages. Although a biopsy is not routinely needed to make the diagnosis, if the diagnosis is questioned a biopsy is sometimes helpful. Included in the differential diagnosis of PIH are disorders such as melasma, exogenous ochronosis, amyloidosis, lichen planus, acanthosis nigricans, erythema dyschromicum perstans, morphea, and tinea versicolor. It is important to check for signs and symptoms and to rule out underlying Addison disease and systemic lupus erythematosus.
PIH presents clinically as asymptomatic macules or patches that range in color from tan to dark brown when there is epidermal melanin, and from blue-gray to gray-brown when there is dermal melanin. Wood’s lamp examination may be helpful when trying to distinguish between epidermal and dermal melanin deposition, with the epidermal melanin becoming accentuated under Wood’s lamp. Epidermal pigment will show fluorescence under Wood’s lamp illumination whereas dermal pigment should not. Mixed and intermediate level pigmentation will show a gradation between the former two. The deeper the pigment, the less fluorescence will occur on Wood’s lamp examination. Often the borders of these lesions are not distinct, due to the distribution in areas of prior inflammation. Often the areas of the hyperpigmentation are clues to the underlying inflammatory etiology. In acne vulgaris the resultant hyperpigmented lesions occur on the head, neck, and upper trunk area, are usually less than 1 cm, and tend to be perifollicular. In lesions resulting from lichen simplex chronicus, areas favored include the ankle and antecubital/popliteal fossae. For lesions due to an atopic dermatitis, in infants the face and forearms are affected whereas older children usually have involvement of the flexural areas. In suspected fixed drug eruptions, circular or nummular lesions are observed usually at a perioral, acral, or genital site. Epidermal hypermelanosis is more responsive to treatment than the dermal counterpart. Postinflammatory epidermal hyperpigmentation should resolve with time once the underlying inflammatory disorder is treated, which may take anywhere from 6 to 12 months. Conversely, dermal hypermelanosis is sometimes permanent.
Both melasma and PIH can be distressing for the patient, and the physician should not minimize the psychosocial impact that these disorders may have on the patient’s social and professional life. These conditions can have major detrimental effects on a patient’s quality of life. Patients may experience feelings of depression and social isolation. Often a feeling of frustration regarding multiple failed treatments as well as frustration with one’s self can arise from experience of both pigmentary disorders.
Explanation and discussion of the pathogenesis, clinical course, and treatment options before embarking on treatment can help to manage expectations and set realistic goals for the patient. Because there is no “quick fix,” patients must be counseled on the time that is required for treatments to take effect so that they themselves do not become discouraged. Because both disorders are characterized by increased epidermal and dermal melanin, production and deposition treatments for both are discussed together. The theme of a stepwise approach combining multiple modalities is emphasized, and prevention especially against ultraviolet radiation exposure is paramount. When approaching both clinical entities a stepwise approach to treatment is recommended while always assessing the patient’s clinical progress, satisfaction, and any adverse events that may occur. It is recommended to start with medical therapy, including topical bleaching agents, retinoids, and low-potency corticosteroids, with treatment durations described in the next section. If adequate resolution is not achieved, treatment can progress to chemical peels and laser therapies, although these should only be done by those who have extensive experience in treating disorders of hyperpigmentation, especially in skin of color. Due to the adverse effects of chemical peeling and laser therapy, including further hyperpigmentation and scarring, one should exercise caution when initiating such therapies.
Medical therapy
For the treatment of PIH specifically the first aspect to be addressed is the treatment of the underlying inflammatory etiology, if still active; this will help halt any further pigmentary alteration. It is acceptable to initiate treatment of the postinflammatory pigmentation concurrently with treatment of the underlying cause; however, the physician must be cognizant that the treatment of the hypermelanosis can exacerbate or cause PIH itself by causing further inflammation. The patient’s assessment of the treatment should always be included at each stage of treatment.
Photoprotection
For both melasma and PIH, photoprotection should be initiated early and throughout the treatment process. Photoprotection is an integral part of the pathogenesis and persistence of melasma and PIH, as continued UV radiation exposure causes melanocyte activation and continued melanin deposition. Broad-spectrum sun protection that covers the UVB and UVA range should be initiated and should be used year-round because daily sun exposure even in winter months, while nominal in some areas, may be a contributory factor. Because the action spectrum of melanogenesis is considered to be in the longer-wavelength UVA range, UVA protection is indispensable. UV protection is of particular importance for those with skin of color and darker phototypes who many not routinely consider that sun protection is necessary—a common misconception among those with darker phenotypes. In fact when data from the 1992 National Health Interview Survey was analyzed, it was found that only a minority of the 1583 African American responders were likely to use sunscreen, wear protective clothing, or stay in the shade. Vitamin D levels may be of concern in people who are using daily sunscreen, especially for patients with darker skin types who are already at risk for vitamin D deficiency. The American Academy of Dermatology has released a consensus statement regarding the use of daily supplementation for people with darker skin phototypes who are at risk for vitamin D deficiency. Through diet and supplementation a total daily dose of 1000 IU for adults is recommended.
Hydroquinone
The next step, and one of the mainstays of the treatment of melasma and PIH, is use of the phenolic compound hydroquinone. This skin-lightening medication acts by blocking the conversion of dihydroxyphenylalanine (DOPA) to melanin through inhibiting the enzyme tyrosinase, the essential step in melanin synthesis. Hydroquinone may also work through inhibiting DNA and RNA synthesis, selective cytotoxicity toward melanocytes, and melanosome degradation by auto-oxidation and phenol oxidases leading to highly reactive oxygen radicals. These reactive substances prevent melanin production within melanosomes and increase degradation of melanosome packages after transfer to adjacent keratinocytes. Hydroquinone produces a gradual reduction of the dyschromia by melanocyte downregulation through prevention of production of melanosomes in the actual transfer of melanin to the keratinocytes. Hydroquinone is most commonly prescribed at a concentration of 4%, but is available up to 10% by prescription and over the counter at 2% concentration. The 4% concentration is the standard therapy for melasma and PIH, and has been used for more than 5 decades. For milder forms of pigment deposition the lower 2% concentration may be effective. Higher 10% concentrations are used for more severe clinical phenotypes. It must be noted though that chronic use of topical hydroquinone, even at 2%, can be associated with the risk of exogenous ochronosis, especially with the darker phototypes. Higher concentrations are also more likely to induce irritation and exogenous ochronosis. This condition is most commonly reported in blacks in South Africa, and there have been a few reports of exogenous ochronosis in the United States. However, there has been an increase in the selling of illicit higher concentrations of hydroquinone at ethnic stores in the United States. When used as a monotherapy, hydroquinone’s effectiveness is seen around 20 weeks of treatment and the efficacy plateaus after 6 months. It is effective when applied twice daily and should be applied to the entire facial area, as excessive lightening of skin not affected by melasma has not been documented. In actuality, localized or so-called spot treatment can lead to “bull’s eye” areas of discoloration.
Hydroquinone and Topical Retinoid
For more moderate to severe melasma topical hydroquinone is combined with a topical retinoid, such as 0.1% tretinoin. The retinol product can be used at night, and this combination can be used for 3 months. Tretinoin and retinol (the precursor to tretinoin) have been shown to be effective in prevention and reversal of photodamage at the molecular level. Pathak and colleagues conducted clinical trials involving 300 Hispanic women with melasma who were treated with various concentrations of hydroquinone formulations. It was concluded that 2% hydroquinone and 0.05% to 0.1% retinoic acid produced the most favorable results. When hydroquinone is combined with a topical retinoid the risk of irritation is increased, and this possibility should be monitored for. If after 3 months the patient does not see improvement than a triple therapy can be initiated. Kligman and colleagues created an early triple-therapy formulation that included 5% hydroquinone, 0.1% tretinoin, and 0.1% dexamethasone, which was highly effective but had inherent problems due to the high concentrations of tretinoin and the fluorinated steroid. Of note, Kligman and colleagues noted poor results when each ingredient was used as monotherapy. A less irritating formulation is that of TriLuma (Galderma, Fort Worth, TX, USA), which contains 4% hydroquinone, 0.05% tretinoin, and 0.01% fluocinolone acetonide. This formulation has been used to treat both melasma and PIH, with successful results. This cream should be tried once a day for 2 months, then treatment should continue with the hydroquinone and retinol product daily for 6 months. After 1 year with no recurrence, maintenance therapy should be initiated with the use of the tretinoin cream at night. If recurrence does occur, the patient should resume the original therapy. Patients with severe melasma who are using the triple therapy should be monitored. Due to the steroid, after 8 weeks of therapy steroid-related side effects such as telangiectasias and steroid acne have been observed.
Mequinol
If hydroquinone is too irritating to the patient, a derivative and alternative is 4-hydroxyanisole or mequinol. Mequinol has been found to be less irritating than hydroquinone. The mechanism of action, while not completely elucidated, is thought to involve a competitive inhibition of tyrosinase. Mequinol is available as a 2% concentration and can be formulated with 0.01% tretinoin. Multiple clinical trials have shown that mequinol can effectively treat solar lentigos in a broad range of skin phototypes; one study compared mequinol 2%/tretinoin 0.01% with hydroquinone 4% and showed that both were equally effective.
Nonphenolic Compounds
Nonphenolic compounds that are used in both melasma and PIH include retinoids, azelaic acid, kojic acid, arbutin, niacinamide, N -acetylglucosamine, ascorbic acid, licorice, and soy.
Retinoids are a widely used medication, and are structural and functional analogues of vitamin A. These agents are effective alone or in combination for both conditions, and can be used as maintenance therapy. Retinoids act via modulation of cell proliferation, differentiation, induction of apoptosis, and expression of anti-inflammatory properties. Tretinoin is all- trans retinoic acid and a first-generation retinoid; its concentration ranges from 0.01% to 0.1% and is often formulated with hydroquinone to act synergistically on aberrant pigment. Callender and colleagues conducted a clinical trial with black patients to test the efficacy and safety of tretinoin 0.1% in the treatment of PIH. Tretinoin was significantly more effective in treating PIH than the control; however, 50% of patients developed retinoid dermatitis. To combat tretinoid dermatitis one can titrate the dosage, use alternate-day dosing, and dilute the tretinoin with a moisturizer base. Griffiths and colleagues reported significant improvement in 68% of melasma patients treated with 0.1% tretinoin in a 40-week trial. The newer, third-generation retinoids, adapalene and tazarotene, have both been shown in clinical trials to effectively treat PIH. Tazarotene is category X.
Azelaic acid is a dicarboxylic acid (1.7-heptanedicarboxylic acid) that occurs naturally and is isolated from Pityriasis versicolor . Azelaic acid inhibits tyrosinase, and inhibits DNA synthesis and mitochondrial enzymes in abnormal and hyperactive melanocytes. This process may be mediated via the inhibition of mitochondrial oxidoreductase activity. Azelaic acid is formulated as a 15% gel normally prescribed for rosacea and a 20% cream commonly used for melasma, PIH, and acne vulgaris. Lowe and colleagues tested azelaic acid in skin types IV to VI with facial PIH or melasma, and demonstrated that it was safe and effective for the treatment of both conditions in these darker skin types. Allergic sensitization and phototoxic reactions are rare, and more common side effects include mild erythema, scaling, and burning.
Kojic acid is another nonphenolic treatment of both melasma and PIH. It is a fungal metabolite of the fungi Acetobacter , Aspergillus , and Penicillium . Kojic acid inhibits tyrosinase and is available in 1% to 4% concentrations, and can also be formulated with other skin-lightening medications such as hydroquinone. Lim and colleagues studied the use of 2% kojic acid combined with hydroquinone for the treatment of melasma, with results showing improvement and efficacy. Therefore, those patients not seeing results from hydroquinone may benefit from the addition of kojic acid to the regime. Kojic acid is becoming a frequent added ingredient in over-the-counter cosmeceutical formulations and thus is becoming an increasing offender for allergic contact dermatitis; therefore, one should not overlook its sensitizing potential.
Arbutin is another naturally derived compound used for hyperpigmentation. It is formulated from the dried leaves of the bearberry shrub, cranberry, pear, or blueberry plants. Arbutin is a derivative of hydroquinone but does not have the same melanotoxic effects. It also inhibits tyrosinase activity but also inhibits melanosome maturation. The effects of arbutin are dose dependent but higher concentrations can cause hyperpigmentation, so this should be monitored for. Synthetic forms have been produced, which show greater tyrosinase inhibition. One study showed that arbutin was effective in treating solar lentigenes in lighter phototypes but failed to have an effect in darker-skinned patients.
Niacinamide is the active derivative of vitamin B3 (niacin), and has been shown in vitro to decrease melanosome transfer from melanocytes to keratinocytes without inhibiting tyrosinase or cell proliferation. It may also interfere with cell signaling pathways. Niacinamide is stable in an array of compounds and is not inactivated by light. It is formulated as 2% to 5% preparations, but its efficacy has not been shown in darker phototypes. Niacinamide has been shown to have efficacy in treating melasma and hyperpigmentation when combined with N -acetylglucosamine, which is a precursor to hyaluronic acid. N -Acetylglucosamine inhibits tyrosinase glycosylation, which is one step in melanin production. It is usually formulated as a 2% compound combined with niacinamide in cosmecuticals.
Ascorbic acid, or vitamin C, is another compound that has been tried for treatment of hyperpigmentation. It is an antioxidant found in various fruits and foods. The mechanism of action in pigment alteration involves interaction with copper ions at the tyrosinase active site as well as reduction of oxidized dopaquinone, which is a substrate in melanin synthesis. There are also some documented anti-inflammatory and photoprotective properties. Ascorbic acid is unstable in many topical preparations so the esterified derivatives, such as ascorbyl-6-palmitate and magnesium ascorbyl phosphate, are used in compounds. There are reports of its efficacy in Latino and Asian patients in the treatment of melasma. Iontophoresis has also been employed to increase the penetration of ascorbic acid into the skin.
Recent studies have shown that flavonoids from licorice roots, such as glabrene and isliquiritigenin, are effective tyrosinase inhibitors and can therefore be used to treat hyperpigmentation. Liquiritin is also a flavonoid available in a 2% cream, which has the ability to cause depigmentation through melanin dispersibility. A study of women with melasma showed efficacy of liquiritin in 80% of patients tested. Mild irritation was seen in only 20% of patients.
Soy proteins are other naturally occurring compounds that have garnered much attention regarding their medicinal purposes. Soy proteins include soybean trypsin inhibitor and Bowman-Birk inhibitor, and act by inhibiting the activation of protease-activated receptor 2 cell receptors on keratinocytes. These keratinocyte receptors mediate the transfer of melanosomes from melanocytes to keratinocytes. Therefore, the action of these soy proteins in the phagocytosis of melanosomes into keratinocytes is reduced and depigmentation occurs. Soy is currently being formulated alone or in combination with retinol and other products in cosmeceuticals, not only for hyperpigmentation but also photodamage.
Medical therapy
For the treatment of PIH specifically the first aspect to be addressed is the treatment of the underlying inflammatory etiology, if still active; this will help halt any further pigmentary alteration. It is acceptable to initiate treatment of the postinflammatory pigmentation concurrently with treatment of the underlying cause; however, the physician must be cognizant that the treatment of the hypermelanosis can exacerbate or cause PIH itself by causing further inflammation. The patient’s assessment of the treatment should always be included at each stage of treatment.
Photoprotection
For both melasma and PIH, photoprotection should be initiated early and throughout the treatment process. Photoprotection is an integral part of the pathogenesis and persistence of melasma and PIH, as continued UV radiation exposure causes melanocyte activation and continued melanin deposition. Broad-spectrum sun protection that covers the UVB and UVA range should be initiated and should be used year-round because daily sun exposure even in winter months, while nominal in some areas, may be a contributory factor. Because the action spectrum of melanogenesis is considered to be in the longer-wavelength UVA range, UVA protection is indispensable. UV protection is of particular importance for those with skin of color and darker phototypes who many not routinely consider that sun protection is necessary—a common misconception among those with darker phenotypes. In fact when data from the 1992 National Health Interview Survey was analyzed, it was found that only a minority of the 1583 African American responders were likely to use sunscreen, wear protective clothing, or stay in the shade. Vitamin D levels may be of concern in people who are using daily sunscreen, especially for patients with darker skin types who are already at risk for vitamin D deficiency. The American Academy of Dermatology has released a consensus statement regarding the use of daily supplementation for people with darker skin phototypes who are at risk for vitamin D deficiency. Through diet and supplementation a total daily dose of 1000 IU for adults is recommended.
Hydroquinone
The next step, and one of the mainstays of the treatment of melasma and PIH, is use of the phenolic compound hydroquinone. This skin-lightening medication acts by blocking the conversion of dihydroxyphenylalanine (DOPA) to melanin through inhibiting the enzyme tyrosinase, the essential step in melanin synthesis. Hydroquinone may also work through inhibiting DNA and RNA synthesis, selective cytotoxicity toward melanocytes, and melanosome degradation by auto-oxidation and phenol oxidases leading to highly reactive oxygen radicals. These reactive substances prevent melanin production within melanosomes and increase degradation of melanosome packages after transfer to adjacent keratinocytes. Hydroquinone produces a gradual reduction of the dyschromia by melanocyte downregulation through prevention of production of melanosomes in the actual transfer of melanin to the keratinocytes. Hydroquinone is most commonly prescribed at a concentration of 4%, but is available up to 10% by prescription and over the counter at 2% concentration. The 4% concentration is the standard therapy for melasma and PIH, and has been used for more than 5 decades. For milder forms of pigment deposition the lower 2% concentration may be effective. Higher 10% concentrations are used for more severe clinical phenotypes. It must be noted though that chronic use of topical hydroquinone, even at 2%, can be associated with the risk of exogenous ochronosis, especially with the darker phototypes. Higher concentrations are also more likely to induce irritation and exogenous ochronosis. This condition is most commonly reported in blacks in South Africa, and there have been a few reports of exogenous ochronosis in the United States. However, there has been an increase in the selling of illicit higher concentrations of hydroquinone at ethnic stores in the United States. When used as a monotherapy, hydroquinone’s effectiveness is seen around 20 weeks of treatment and the efficacy plateaus after 6 months. It is effective when applied twice daily and should be applied to the entire facial area, as excessive lightening of skin not affected by melasma has not been documented. In actuality, localized or so-called spot treatment can lead to “bull’s eye” areas of discoloration.
Hydroquinone and Topical Retinoid
For more moderate to severe melasma topical hydroquinone is combined with a topical retinoid, such as 0.1% tretinoin. The retinol product can be used at night, and this combination can be used for 3 months. Tretinoin and retinol (the precursor to tretinoin) have been shown to be effective in prevention and reversal of photodamage at the molecular level. Pathak and colleagues conducted clinical trials involving 300 Hispanic women with melasma who were treated with various concentrations of hydroquinone formulations. It was concluded that 2% hydroquinone and 0.05% to 0.1% retinoic acid produced the most favorable results. When hydroquinone is combined with a topical retinoid the risk of irritation is increased, and this possibility should be monitored for. If after 3 months the patient does not see improvement than a triple therapy can be initiated. Kligman and colleagues created an early triple-therapy formulation that included 5% hydroquinone, 0.1% tretinoin, and 0.1% dexamethasone, which was highly effective but had inherent problems due to the high concentrations of tretinoin and the fluorinated steroid. Of note, Kligman and colleagues noted poor results when each ingredient was used as monotherapy. A less irritating formulation is that of TriLuma (Galderma, Fort Worth, TX, USA), which contains 4% hydroquinone, 0.05% tretinoin, and 0.01% fluocinolone acetonide. This formulation has been used to treat both melasma and PIH, with successful results. This cream should be tried once a day for 2 months, then treatment should continue with the hydroquinone and retinol product daily for 6 months. After 1 year with no recurrence, maintenance therapy should be initiated with the use of the tretinoin cream at night. If recurrence does occur, the patient should resume the original therapy. Patients with severe melasma who are using the triple therapy should be monitored. Due to the steroid, after 8 weeks of therapy steroid-related side effects such as telangiectasias and steroid acne have been observed.
Mequinol
If hydroquinone is too irritating to the patient, a derivative and alternative is 4-hydroxyanisole or mequinol. Mequinol has been found to be less irritating than hydroquinone. The mechanism of action, while not completely elucidated, is thought to involve a competitive inhibition of tyrosinase. Mequinol is available as a 2% concentration and can be formulated with 0.01% tretinoin. Multiple clinical trials have shown that mequinol can effectively treat solar lentigos in a broad range of skin phototypes; one study compared mequinol 2%/tretinoin 0.01% with hydroquinone 4% and showed that both were equally effective.
Nonphenolic Compounds
Nonphenolic compounds that are used in both melasma and PIH include retinoids, azelaic acid, kojic acid, arbutin, niacinamide, N -acetylglucosamine, ascorbic acid, licorice, and soy.
Retinoids are a widely used medication, and are structural and functional analogues of vitamin A. These agents are effective alone or in combination for both conditions, and can be used as maintenance therapy. Retinoids act via modulation of cell proliferation, differentiation, induction of apoptosis, and expression of anti-inflammatory properties. Tretinoin is all- trans retinoic acid and a first-generation retinoid; its concentration ranges from 0.01% to 0.1% and is often formulated with hydroquinone to act synergistically on aberrant pigment. Callender and colleagues conducted a clinical trial with black patients to test the efficacy and safety of tretinoin 0.1% in the treatment of PIH. Tretinoin was significantly more effective in treating PIH than the control; however, 50% of patients developed retinoid dermatitis. To combat tretinoid dermatitis one can titrate the dosage, use alternate-day dosing, and dilute the tretinoin with a moisturizer base. Griffiths and colleagues reported significant improvement in 68% of melasma patients treated with 0.1% tretinoin in a 40-week trial. The newer, third-generation retinoids, adapalene and tazarotene, have both been shown in clinical trials to effectively treat PIH. Tazarotene is category X.
Azelaic acid is a dicarboxylic acid (1.7-heptanedicarboxylic acid) that occurs naturally and is isolated from Pityriasis versicolor . Azelaic acid inhibits tyrosinase, and inhibits DNA synthesis and mitochondrial enzymes in abnormal and hyperactive melanocytes. This process may be mediated via the inhibition of mitochondrial oxidoreductase activity. Azelaic acid is formulated as a 15% gel normally prescribed for rosacea and a 20% cream commonly used for melasma, PIH, and acne vulgaris. Lowe and colleagues tested azelaic acid in skin types IV to VI with facial PIH or melasma, and demonstrated that it was safe and effective for the treatment of both conditions in these darker skin types. Allergic sensitization and phototoxic reactions are rare, and more common side effects include mild erythema, scaling, and burning.
Kojic acid is another nonphenolic treatment of both melasma and PIH. It is a fungal metabolite of the fungi Acetobacter , Aspergillus , and Penicillium . Kojic acid inhibits tyrosinase and is available in 1% to 4% concentrations, and can also be formulated with other skin-lightening medications such as hydroquinone. Lim and colleagues studied the use of 2% kojic acid combined with hydroquinone for the treatment of melasma, with results showing improvement and efficacy. Therefore, those patients not seeing results from hydroquinone may benefit from the addition of kojic acid to the regime. Kojic acid is becoming a frequent added ingredient in over-the-counter cosmeceutical formulations and thus is becoming an increasing offender for allergic contact dermatitis; therefore, one should not overlook its sensitizing potential.
Arbutin is another naturally derived compound used for hyperpigmentation. It is formulated from the dried leaves of the bearberry shrub, cranberry, pear, or blueberry plants. Arbutin is a derivative of hydroquinone but does not have the same melanotoxic effects. It also inhibits tyrosinase activity but also inhibits melanosome maturation. The effects of arbutin are dose dependent but higher concentrations can cause hyperpigmentation, so this should be monitored for. Synthetic forms have been produced, which show greater tyrosinase inhibition. One study showed that arbutin was effective in treating solar lentigenes in lighter phototypes but failed to have an effect in darker-skinned patients.
Niacinamide is the active derivative of vitamin B3 (niacin), and has been shown in vitro to decrease melanosome transfer from melanocytes to keratinocytes without inhibiting tyrosinase or cell proliferation. It may also interfere with cell signaling pathways. Niacinamide is stable in an array of compounds and is not inactivated by light. It is formulated as 2% to 5% preparations, but its efficacy has not been shown in darker phototypes. Niacinamide has been shown to have efficacy in treating melasma and hyperpigmentation when combined with N -acetylglucosamine, which is a precursor to hyaluronic acid. N -Acetylglucosamine inhibits tyrosinase glycosylation, which is one step in melanin production. It is usually formulated as a 2% compound combined with niacinamide in cosmecuticals.
Ascorbic acid, or vitamin C, is another compound that has been tried for treatment of hyperpigmentation. It is an antioxidant found in various fruits and foods. The mechanism of action in pigment alteration involves interaction with copper ions at the tyrosinase active site as well as reduction of oxidized dopaquinone, which is a substrate in melanin synthesis. There are also some documented anti-inflammatory and photoprotective properties. Ascorbic acid is unstable in many topical preparations so the esterified derivatives, such as ascorbyl-6-palmitate and magnesium ascorbyl phosphate, are used in compounds. There are reports of its efficacy in Latino and Asian patients in the treatment of melasma. Iontophoresis has also been employed to increase the penetration of ascorbic acid into the skin.
Recent studies have shown that flavonoids from licorice roots, such as glabrene and isliquiritigenin, are effective tyrosinase inhibitors and can therefore be used to treat hyperpigmentation. Liquiritin is also a flavonoid available in a 2% cream, which has the ability to cause depigmentation through melanin dispersibility. A study of women with melasma showed efficacy of liquiritin in 80% of patients tested. Mild irritation was seen in only 20% of patients.
Soy proteins are other naturally occurring compounds that have garnered much attention regarding their medicinal purposes. Soy proteins include soybean trypsin inhibitor and Bowman-Birk inhibitor, and act by inhibiting the activation of protease-activated receptor 2 cell receptors on keratinocytes. These keratinocyte receptors mediate the transfer of melanosomes from melanocytes to keratinocytes. Therefore, the action of these soy proteins in the phagocytosis of melanosomes into keratinocytes is reduced and depigmentation occurs. Soy is currently being formulated alone or in combination with retinol and other products in cosmeceuticals, not only for hyperpigmentation but also photodamage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree