CHAPTER 6 TRAM Flap Breast Reconstruction
Introduction
Breast reconstruction after mastectomy had its primitive beginning with an implant placed subcutaneously in a delayed procedure. The concept of immediate reconstruction was rejected for numerous unsubstantiated reasons, notably that the patient should live with the deformity and that early cancer recurrence would be masked by the reconstruction. Goin and Goin1 advocated immediate reconstruction and emphasized the added benefit of contralateral prophylactic mastectomy when appropriate. Many specialists who could potentially refer their patients for reconstruction did not because of these unfounded concerns. In the late 1970s, musculocutaneous flaps were introduced, offering first the latissimus dorsi.2 Several years later, Hartrampf et al3 presented the rectus abdominis procedure which offered completely autologous reconstruction. These new techniques, together with tissue expanders, now achieved superior aesthetic results with a high degree of reliability. Despite these facts, convincing the oncologic surgeons to offer their patients delayed or immediate reconstruction was very difficult. With slow acceptance of delayed procedures, it took much longer for the immediate procedure to be recognized. Today the surgical armamentarium of flaps and tissue expander/implants makes the prospects of mastectomy for patients much easier to accept. For surgeons recommending the option of breast conservation versus mastectomy, the prospect of a satisfactory aesthetic result makes mastectomy more acceptable, if indicated. This includes those patients with small breasts or tumors located in the upper quadrants where the aesthetic result after lumpectomy and radiation often produce less than ideal results.
Indications
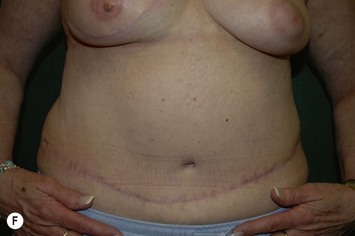
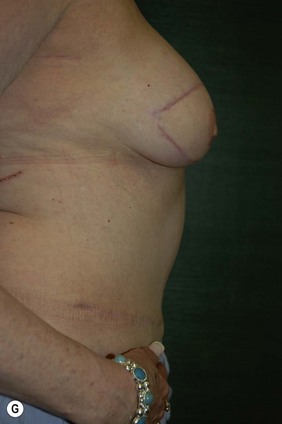
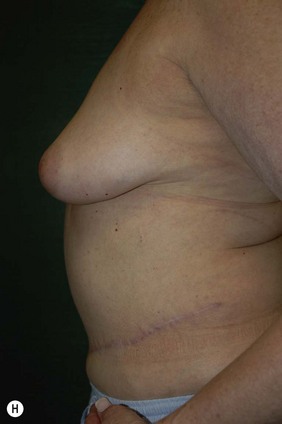
The decision for TRAM reconstruction depends upon the patient’s appropriate anatomy and medical condition. Significant obesity and/or an associated pannus of redundant skin may compromise the circulation to the abdominal wall or TRAM. Abdominal wall scars may compromise the circulation of the abdominal skin flap resulting in necrosis and delayed healing.4 The subcostal scar resulting from an open cholecystectomy is associated with division of the rectus muscle precluding its use as a pedicle on that side. A modification of the skin incision on the right abdomen to include the subcostal scar with the skin island placed higher eliminates the problem of an ischemic area below the scar, a potential cause of abdominal flap necrosis. This modification does not interfere with developing a satisfactory left-sided pedicle.5 Vertical midline scars do not allow use of tissue across the scar unless a bilateral pedicle is employed for unilateral reconstruction. Recently, Mustoe has demonstrated that a delay procedure allows survival of tissue across a vertical midline scar with a unilateral pedicle.6 Suprapubic scars do not pose a problem to the blood supply of either donor site or the TRAM musculocutaneous unit. With a large percentage of patients having undergone prior gynecologic procedures, the major problem encountered has been a more technically difficult dissection due to scarring. In one case, bowel adherent to the rectus muscle in an unrecognized midline infraumbilical hernia required a segmental small bowel resection when the gut wall was injured during a difficult dissection. A right lower quadrant appendectomy scar limits the use of tissue lateral to the scar or favors the use of a left sided pedicle as an alternative. Subjects who are very thin are still viable candidates if they have adequate redundant skin to create a TRAM island and allow closure of the donor site. Here, a prosthesis may be added immediately or at the time of nipple–areola reconstruction, which is the author’s preference.
Those patients with significant lumbosacral disease, that is, spondylolisthesis, are thought to be further compromised if the rectus muscle is sacrificed. Severe asthma or chronic obstructive pulmonary disease may compromise the postoperative recovery, but do not contraindicate the procedure. Ischemic heart disease may preclude a long surgery and anesthesia. Cardiac decompensation is a pertinent consideration for patients who have received prior treatment with cardiotoxic chemotherapeutic agents. Insulin-dependent diabetics have an increased risk of complications in general, but of particular concern is the viability of the abdominal wall where ischemia of the flap may result in fat or skin necrosis and wound dehiscence. Similar potential problems apply to smokers7 and obese patients.8
The choice of a pedicled TRAM over other autologous methods of reconstruction or prosthesis-based techniques depends upon many objective factors as described above. The subjective reasons to choose a particular procedure often involves the patient, referring oncologic surgeon or other healthcare provider. Experience with other patients, those of friends or family also often play a role in decision making. Because autologous reconstruction arguably offers the best result, it is the author’s first choice on the reconstructive ladder. Likewise, immediate reconstruction should be considered unless postoperative radiation therapy to the chest wall is certain or there is a possibility that a contralateral mastectomy is considered but refused by the patient or is not practical at the time. A tissue expander may be placed as a temporary method of preserving the mastectomy flaps as a spacer and leave the option open for later autologous reconstruction.9 Genetic testing has significantly increased the number of patients opting for a prophylactic mastectomy of the opposite breast when diagnosed with cancer. The importance of family history in BRCA negative patients has also made prophylactic uni- and bilateral mastectomy and reconstruction a common occurrence.10 Likewise, those patients with strong family history, positive genetic markers or concerns about cancer detection are presenting for bilateral mastectomy without a cancer diagnosis.11–13 Because the abdominal donor site can only be used once, risk of future disease in the opposite breast must be considered as part of the treatment options and informed consent.
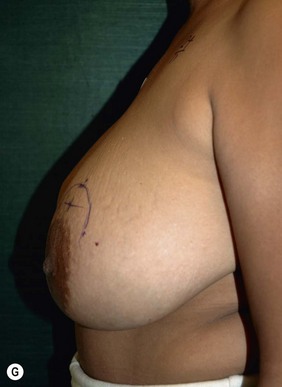
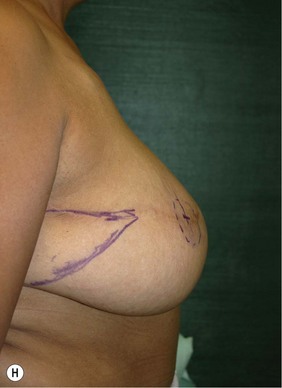
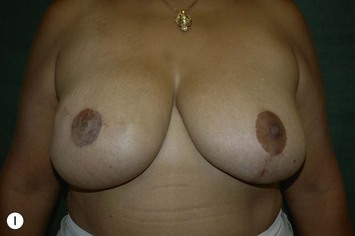
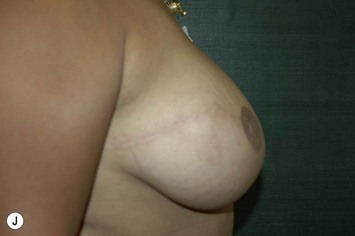
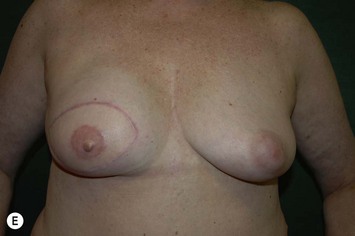
Planning mastectomy incisions is a collaborative effort between the oncologic and reconstructive surgeon to consider the many options. The position of the tumor and its relation to the nipple–areola is most important as the skin incision will be determined by their location. Many surgeons will include the biopsy site in the skin incision leading to additional skin flap sacrifice. The concept of skin sparing mastectomy has many interpretations. Preservation of the breast skin usually facilitates better aesthetic outcomes. When there is enough skin to allow a completely de-epithelialized TRAM island, minimal scarring usually results and there is no mismatch of skin color or contour. A transversely oriented scar can later be disguised if the nipple reconstruction punctuates it. Similarly, when only the nipple–areola is sacrificed, the scar is minimal as reconstruction of the nipple–areola will completely obliterate most or all of it. If the breast is large enough and the tumor position allows preservation of the upper breast skin, a Wise pattern (keyhole) mastectomy may be employed.14 This approach allows contouring to reduce lateral fullness and very acceptable incision placement. The secondarily reconstructed nipple can usually be placed at or near the apex of the vertical limb of the inverted ‘T.’ A vertical mammaplasty pattern may also be useful in a skin sparing mastectomy.15,16
Operative Technique I: Planning
Vascular anatomy
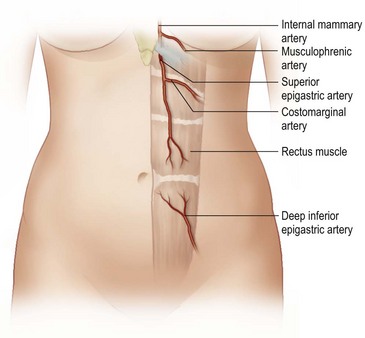
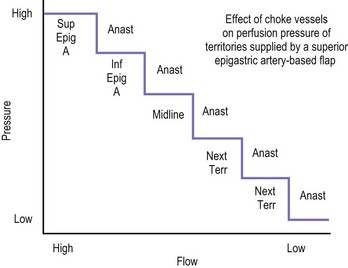
The design of the cutaneous component of a pedicle flap must take advantage of the best blood supply while utilizing the most satisfactory tissue to create a breast mound. The internal mammary artery descends subcostally dividing into the musculophrenic and deep superior epigastric branches. The musculophrenic sends branches to the intercostal vessels. This costomarginal anastomotic circulation is an alternate one when the internal mammary is divided as a result of previous surgery. The deep superior epigastric artery emerges under the medial costal margin and enters the deep surface of the muscle with its accompanying veins. The vessels course within the body of the muscle. Above the level of the umbilicus, the vessels become a web of choke vessels, which anastamose with the vascular supply from the deep inferior epigastric system.17 Since the deep inferior epigastric artery that branches from the internal iliac is the dominant pedicle, there is circulatory compromise when the artery and its two venae comitantes are divided to allow pedicle transfer. This is because the skin island is located over the angiosome of the deep inferior epigastric artery and veins. The venous valves prevent flow superiorly until dilatation secondary to venous congestion renders them incompetent (Fig. 6.1). Understanding the flow dynamics within the muscle and subcutaneous components of the flap are best explained in Moon and Taylor’s diagrammatic representation of the ‘staircase effect’ (Fig. 6.2) Venous return is compromised more often than arterial and is manifested as venous congestion. When flap circulation is compromised, a satisfactory solution is decompression of one of the veins. This maneuver is described later in this chapter. Moon and Taylor18 studied the rectus circulation and describe three patterns of supply from the deep epigastric artery. The most common type branches into two vessels just below the arcuate line. The inferior epigastric pedicle most often enters the deep side of the muscle from the lateral side. Perforating vessels are found in two rows just medial and lateral to the edges of the rectus fascia. There are no perforators below the arcuate line. The skin overlying the rectus muscle is supplied by perforators which pierce the fascia and arborize within the subcutaneous fat. These perforating vessels are largest in number in the periumbilical region and are a prime consideration in designing the skin island to include this region. Slavin19 designed a skin island, which is centered above and below the umbilicus. While this design may result in better arterial supply, it may not allow the advantage of utilizing the best subcutaneous tissue and may leave too short a pedicle to allow adequate rotation. This design results in a surgical scar higher on the abdominal wall, which is more noticeable, thus less desirable.
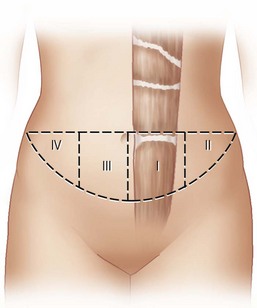
The available skin and fat are considered in four vascular zones (Fig. 6.3). They are numbered in decreasing order of blood supply flow: I–IV. Zone I lies directly over the muscle and has the best circulation with zone II lateral to zone I on the ipsilateral side. Across the midline, zone III has decreased pressure and as a result, its viability is often questionable and must be carefully assessed intraoperatively. Zone IV should not be considered as its viability is always poor. Some authors have labeled zone II as the segment across the midline and zone III ipsilateral next to zone I, but that implies a misleading stepwise progression of blood supply and viability. When a bilateral procedure is performed, each flap is composed of zone I and II. The impact of a vertical infraumbilical scar is discussed earlier.
Operative Technique II: Surgical
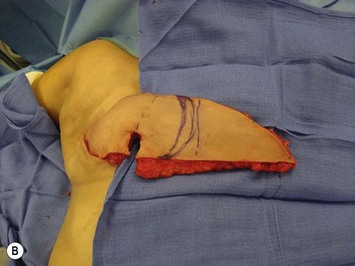
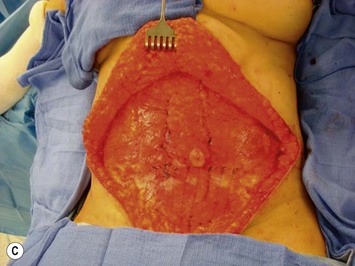
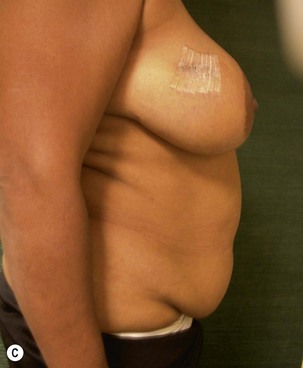
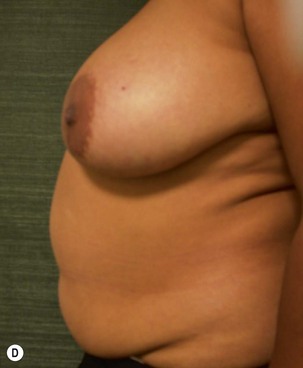
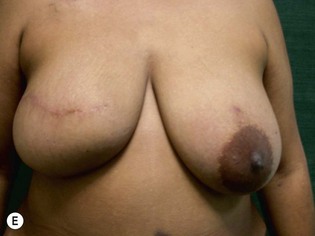
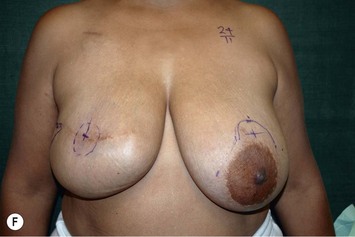
Unilateral procedure (Figs 6.4–6.6)
It is important that the operating table can be flexed to facilitate abdominal wall closure and that it is padded appropriately for the length of the procedure to prevent undue pressure on bony prominences. Sequential compression devices, a catheter in the urinary bladder, and antibiotic prophylaxis are essential. Pharmacologic DVT prophylaxis must be considered for high risk patients.20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree