Category
Drug name
Specific enzyme(s) inhibited
Calcineurin
Cyclosporine
Calcineurin inhibitors
Antimetabolites/purine analogues
Azathioprine
None
Mycophenolate mofetil
Inosine monophosphate dehydrogenase
Antimetabolites/folate antagonists
Methotrexate
Dihydrofolate reductase, Thymidylate synthetase
Dapsone
Dihydropteroate synthetase, Myeloperoxidase
Alkylating agents
Cyclophosphamide
None
Antimalarials
Hydroxychloroquine
None
The emphasis here is on the primary mechanisms of action, particularly as these mechanisms relate to common indications, significant adverse effects, and drug interactions. The discussion of these indications, adverse effects, and drug interactions is brief, emphasizing those with the greatest clinical relevance for the practicing dermatologist. Chapters and reviews that provide greater detail of potential interest are cited.
The drugs above can be divided into two broad groups: immunosuppressive and anti-inflammatory. But many drugs overlap the categories; methotrexate, for example, has both immunosuppressive and anti-inflammatory mechanisms. Thus, considering the drugs discussed as “immune modulating” in a broad sense is a very reasonable approach.
Cyclosporine
Mechanisms of Action [1]
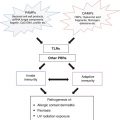
The most established role of cyclosporine (CsA) in psoriasis and other immune-mediated dermatoses is its effect on T lymphocytes [2]. Calcineurin is a calcium- and calmodulin-dependent enzyme that is of central importance to the T-cell amplification of the immune response, in particular inducing increased levels of interleukin-2 (IL-2) (Fig. 47.1). Cyclosporine inhibits calcineurin, which leads to reduced activity of the transcription factor, nuclear factor of activated T cells 1 (NFAT-1) [3]. This transcription factor is important in regulating transcription of a number of cytokine genes, the most significant being IL-2. Because IL-2 causes the proliferation of activated helper T cells (CD4) and cytotoxic T cells (CD8), impaired IL-2 production leads to a decline in the number of activated CD4 and CD8 cells in the epidermis and dermis.
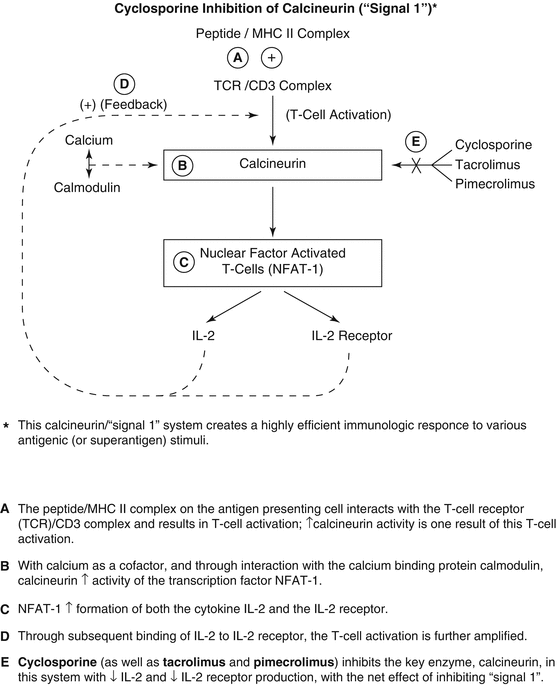

Fig. 47.1
Cyclosporine inhibition of calcineurin*. IL interleukin, MHC major histocompatibility complex
In addition, CsA inhibits the production of interferon-γ, which in turn downregulates intercellular adhesion molecule 1 (ICAM-1) production. ICAM-1 is expressed on the surface of various cells such as keratinocytes and dermal capillary endothelium, playing an important role in the immune process by affecting trafficking of various inflammatory cells. Finally it is important to note that cyclosporine is both a cytochrome P-450 (CYP) 3A4 substrate and inhibitor, explaining many of the numerous potential drug interactions involving cyclosporine.
Clinical Applications of Cyclosporine Mechanisms [1]
Common Indications
- 1.
Psoriasis, atopic dermatitis, refractory urticaria; T-cell inhibition through cyclosporine inhibition of calcineurin and resultant reduced NFAT-1 production.
- 2.
Pyoderma gangrenosum, immunobullous dermatoses (pemphigus and pemphigoid), autoimmune connective tissue diseases (dermatomyositis), and many others; additional dermatoses in which the T cell has a key role in the pathogenesis.
Significant Adverse Effects
- 1.
Renal disease and resultant hypertension; kidney has relatively high levels of calcineurin.
- 2.
Neurologic adverse effects (such as tremors, headache, paresthesias); various neurologic cell types likewise with relatively high levels of calcineurin.
Drug Interactions
- 1.
Macrolides (erythromycin > clarithromycin), azole antifungals (ketoconazole > itraconazole); cyclosporine toxicity due to these CYP3A4 inhibitors.
- 2.
Rifampin (and other “enzyme inducers”); loss of cyclosporine efficacy due to CYP3A4 inducers.
- 3.
Statins such as simvastatin, atorvastatin, lovastatin > rosuvastatin, fluvastatin. CYP3A4 inhibition increasing the risk of rhabdomyolysis from these statins. Pravastatin has no CYP metabolism and is the best choice to use with cyclosporine.
- 4.
Numerous other CYP-based drug interactions (see pertinent table in Lee and Koo [1]).
Azathioprine
Mechanisms of Action [4]
Azathioprine’s active metabolites are 6-thioguanine (6-TG) monophosphate and other 6-TG metabolites; these metabolites are structurally very similar to the endogenous purines adenine and guanine. This structural similarity to the endogenous purines allows these 6-TG metabolites to be incorporated into DNA and RNA, inhibiting purine metabolism and cell division [5, 6]. T-cell–mediated function is depressed, and antibody production is diminished in the B cell [7]. Azathioprine also decreases the number of Langerhans cells (and the ability to present antigens) and other antigen-presenting cells in the skin [8].
Azathioprine is a prodrug which is rapidly converted to 6-mercaptopurine (6-MP) upon absorption. There are three metabolic pathways which subsequently metabolize 6-MP: (1) hypoxanthine- guanine phosphoribosyltransferase (HGPRT), which leads to formation of the active 6-TG metabolites; (2) thiopurine methyltransferase (TPMT), which leads to inactive metabolites; and (3) xanthine oxidase (XO), which also leads to inactive metabolites (Fig. 47.2).
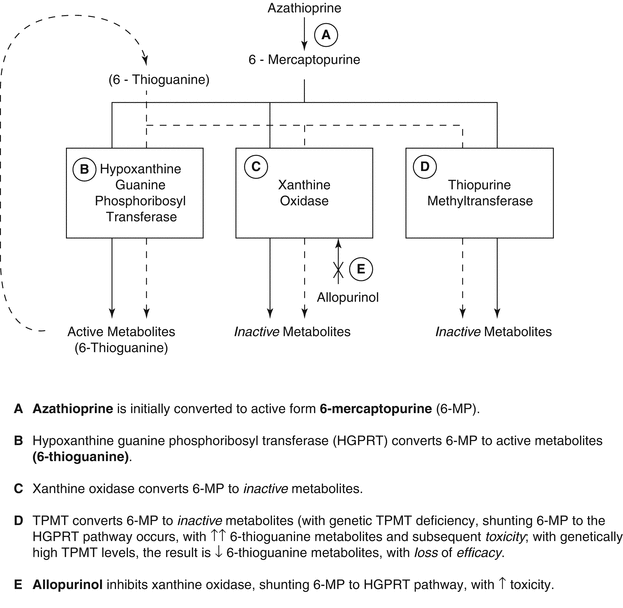

Fig. 47.2
Azathioprine metabolic pathways
The degradative pathways TPMT and XO may indirectly alter the levels of 6-TG metabolites in different ways. The TPMT activity is reduced or absent in certain patients with a genetic polymorphism, while XO can be inhibited by drug interactions with azathioprine involving allopurinol and febuxostat [9, 10]. The net effect of these clinical scenarios is the risk of significant myelosuppression due to increased 6-TG metabolites. In contrast, patients with high levels of TPMT have relatively low levels of the active 6-TG metabolites and may be therapeutically underdosed with azathioprine [11, 12].
It is important to note that significant variation of TPMT activity is present when comparing different ethnic groups. Genetic testing (genotype) for TPMT is readily available, and can generally at least verify that the patient is a homozygote for high activity (TPMT 1*/1*) or a heterozygote for high activity (TPMT 1*/other allele). Functional assays of thiopurine methyltransferase red blood cell (RBC) activity are also available and widely utilized [11].
Clinical Applications of Azathioprine Mechanisms [4]
Common Indications
- 1.
Pemphigus and pemphigoid spectrums; azathioprine inhibition of antibody production.
- 2.
Cutaneous vasculitis (refractory), pyoderma gangrenosum, severe atopic dermatitis, chronic actinic dermatitis, sarcoidosis; inhibition of T-cell function.
Significant Adverse Effects
- 1.
Carcinogenicity including non-Hodgkin’s B- cell lymphomas (this does not appear to be a significant risk with dermatologic conditions with immunologic etiologies; no doubt is a risk with organ transplantation patients), due to altered immune surveillance resulting from azathioprine immunosuppressive properties.
- 2.
Myelosuppression, especially with genetically decreased TPMT levels, shunting 6-MP increasingly to HGPRT pathway, resulting in increased 6-TG metabolites.
- 3.
Opportunistic infections (theoretically; in reality opportunistic infections are very uncommon with azathioprine use for dermatologic indications), due to altered immune surveillance resulting from T-cell and B-cell effects of azathioprine.
- 4.
Gastrointestinal (GI) adverse effects, such as rapidly dividing cells given that azathioprine a cell-cycle–specific antimetabolite.
Drug Interactions
Allopurinol; XO inhibition by allopurinol shunts increased amounts of 6-MP through the HGPRT pathway, leading to increased 6-TG metabolites.
Mycophenolate Mofetil
Mechanisms of Action [13]
Mycophenolate mofetil is rapidly converted to mycophenolic acid (MPA). On systemic absorption, MPA is inactivated by glucuronidation in the liver, and subsequently converted back to its active form by β-glucuronidase within the epidermis and gastrointestinal tract.
Mycophenolic acid has a key role in immune-mediated skin diseases by inhibiting de novo purine synthesis. It is a noncompetitive inhibitor of inosine monophosphate dehydrogenase (Fig. 47.3). Cells relying on de novo purine synthesis, rather than the purine salvage pathway, are preferentially affected. Therefore, the proliferative responses of T lymphocytes and B lymphocytes, which lack the purine salvage pathway, are blocked [14, 15]. Virtually all other cell lines in the body can utilize the purine salvage pathway, which lessens the inhibitory effects of this drug on nonimmunologic cells. Mycophenolic acid also leads to decreased levels of immunoglobulins and decreased delayed-type hypersensitivity responses [16].
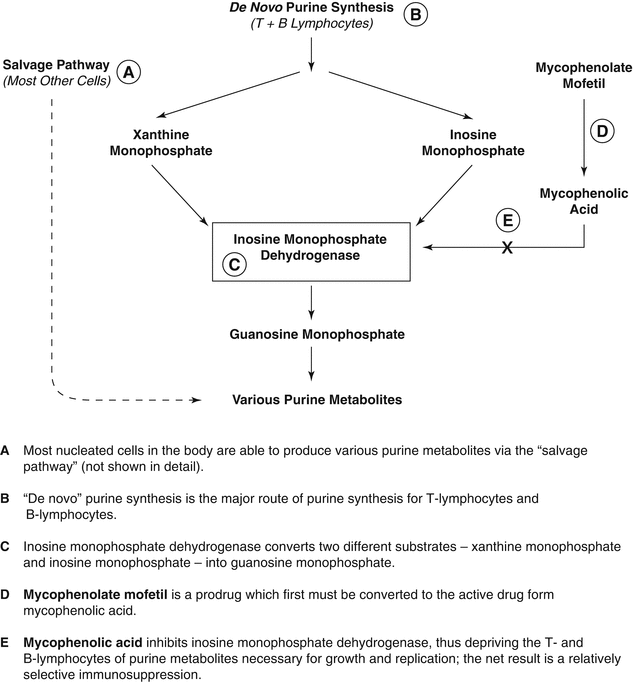

Fig. 47.3
Mycophenolate mofetil inhibition of de novo purine synthesis
Clinical Applications of Mycophenolate Mofetil Mechanisms [13]
Common Indications
- 1.
Pyoderma gangrenosum, psoriasis; relatively selective T-cell inhibition by mycophenolate mofetil.
- 2.
Immunobullous dermatoses (including cicatricial pemphigoid, pemphigus vulgaris, others); relatively selective B-cell inhibition by this drug.
Significant Adverse Effects
- 1.
Gastrointestinal adverse effects; antimetabolite, cell-cycle specific effects on rapidly dividing cells theoretically; however, these cells in the GI tract largely have salvage pathway for purine metabolism.
- 2.
Relatively small number of serious adverse effects; probably the result of the selectivity for the mechanism, with effects primarily on T- and B-lymphocyte subsets.
Drug Interactions
Azathioprine, methotrexate, tumor necrosis factor (TNF) inhibitors; pharmacodynamic interaction with potential for increased myelosuppression or opportunistic infections.
Methotrexate
Mechanisms of Action [17]
Methotrexate competitively and reversibly binds to dihydrofolate reductase, which prevents the conversion of dihydrofolate to tetrahydrofolate (Fig. 47.4). Tetrahydrofolate is a necessary cofactor in the synthesis of thymidylate and purine nucleotides needed for DNA and RNA synthesis. A partially reversible, competitive inhibition of thymidylate synthetase also occurs within 24 h after administration of methotrexate. Methotrexate is an antimetabolite specific for the S phase (synthesis, including DNA synthesis) of cell division, with the greatest impact on rapidly dividing cells. Cells of the GI tract and various hematologic cells are rapidly dividing groups of cells that are particularly sensitive to methotrexate inhibition of cell division.
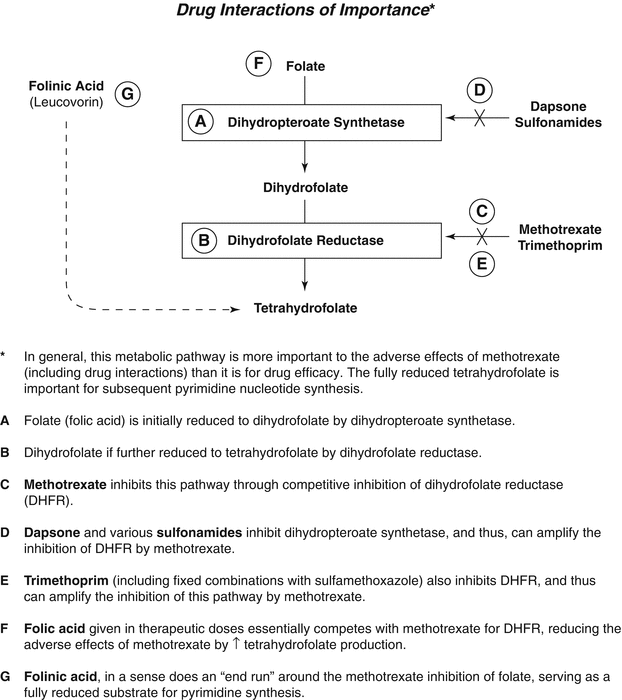

Fig. 47.4
Methotrexate and folate metabolism
Immunosuppression probably occurs because of inhibition of DNA synthesis in immunologically competent cells. The drug can suppress primary and secondary antibody responses as well [18, 19]. There is no significant effect on delayed-type hypersensitivity. An additional effect of MTX is to block migration of activated T cells into various tissues through alteration of various adhesion molecules [20]. The drug’s anti-inflammatory effects are likely predominantly mediated by local increases in adenosine concentration, which has inherent anti-inflammatory properties. This increased adenosine production is the result of complex interactions with aminoimidazole carboxamide ribonucleotide (AICAR) transformylase and ecto-5′-nucleotidase [21].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree