© Springer International Publishing Switzerland 2017
Anthony A. Gaspari, Stephen K. Tyring and Daniel H. Kaplan (eds.)Clinical and Basic Immunodermatology10.1007/978-3-319-29785-9_4646. Topical Immune Response Modifiers: Antiinflammatories
(1)
Department of Dermatology, University Hospital Münster, Von-Esmarch-Str. 58, Münster, D-48149, Germany
(2)
Department of Dermatology, University College Dubin, Charles Institute of Dermatology, Dublin, Ireland
(3)
Department of Dermatology and UCD Charles Institute for Dermatology, University College Dublin, Dublin, Ireland
Abstract
In recent years, major findings such as blockade of the calcineurin pathway in T lymphocytes led to the identification of novel targets for the treatment of inflammatory skin diseases. The first systemic specific calcineurin inhibitor (CI) for the treatment of inflammatory skin diseases was cyclosporin A (CsA), which demonstrated efficacy both in psoriasis and atopic dermatitis (AD). Because of its systemic adverse effects and the inability to generate a topical CsA compound, there still exists a need for better immunomodulatory agents. Later, the calcineurin inhibitor tacrolimus (FK506) was successfully approved as an efficient topical drug. Subsequently a second calcineurin inhibitor (pimecrolimus, ASM 981) was developed and approved for the topical treatment of atopic dermatitis. Both CIs have been shown to function as effective inhibitors of inflammatory responses in the skin. In addition to T cells, they appear to target other inflammatory cells including mast cells, eosinophils and basophils, blocking cytokine production and reducing associated pruritus. This chapter focuses on topical CIs and briefly discusses recent promising developments of topical antiinflammatory agents.
Keywords
AntiinflammatoriesTopical calcineurin inhibitorsTacrolimusPimecrolimusCITH1 and TH2 cytokinesPharmacokinetic StudiesAtopic-induced pruritusPruritusKey Points
New antiinflammatory therapies against atopic dermatitis and other inflammatory skin diseases have become available in recent years.
Topical calcineurin inhibitors include tacrolimus and pimecrolimus.
Topical calcineurin inhibitors exert a potent antiinflammatory activity with a low immunosuppressive potential and no induction of skin atrophy.
In recent years, major findings such as blockade of the calcineurin pathway in T lymphocytes led to the identification of novel targets for the treatment of inflammatory skin diseases. The first systemic specific calcineurin inhibitor (CI) for the treatment of inflammatory skin diseases was cyclosporin A (CsA), which demonstrated efficacy both in psoriasis and atopic dermatitis (AD). Because of its systemic adverse effects and the inability to generate a topical CsA compound, there still exists a need for better immunomodulatory agents. Later, the calcineurin inhibitor tacrolimus (FK506) was successfully approved as an efficient topical drug [1]. Subsequently a second calcineurin inhibitor (pimecrolimus, ASM 981) was developed and approved for the topical treatment of atopic dermatitis. Both CIs have been shown to function as effective inhibitors of inflammatory responses in the skin. In addition to T cells they appear to target other inflammatory cells including mast cells, eosinophils and basophils, blocking cytokine production and reducing associated pruritus [2, 125]. This chapter focuses on topical CIs and briefly discusses recent promising developments of topical antiinflammatory agents.
Besides CIs, glucocorticoids (GCs) are widely used topical antiinflammatory agents in dermatology. However in addition to their therapeutic benefits their adverse effects have also become apparent [13–17].The use of glucocorticosteroids is discussed in Chapter 48 and so this chapter focuses on the impact of CIs as antiinflammatory agents in dermatology.
Mechanism of Calcineurin Inhibition by Tacrolimus and Pimecrolimus
Tacrolimus and pimecrolimus are ascomycin macrolactam derivatives produced by bacteria. While tacrolimus is a product of Streptomyces tsukubaensis, pimecrolimus was generated from Streptomyces hygroscopicus var. ascomycetus. Both bind, albeit with different affinity, to a cytosolic immunophilin receptor, defined as FK-binding protein-12 (macrophilin-12) [9]. After binding, the macrophilin complex associated with either tacrolimus or pimecrolimus inhibits a calcium-dependent serine-threonine phosphatase, defined as calcineurin. Thereby, dephosphorylation and nuclear translocation of a cytosolic transcription factor, the nuclear factor of activated T-cell protein (NF-ATp) is inhibited [6, 18]. Therefore, both tacrolimus and pimecrolimus can be defined as CIs.
In Vitro Effects
Tacrolimus and pimecrolimus inhibit the production of TH1 and TH2 cytokines such as interleukin-2 (IL-2), IL-4, IL-8, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) in vitro. The generation of granulocyte-macrophage colony-stimulating factor (GM-CSF) can also be blocked by these compounds. Pimecrolimus is also capable of supporting anti-inflammatory effects on T-helper-2 (Th2) cells by downregulating IL-5 and IL-13 in CD4+ as well as in CD8+ T cells [19], diminishing the number of CD1+ inflammatory dendritic cells from the epidermis [19, 20], and stimulating apoptosis in skin T cells but not in Langerhans cells [20]. In mast cells, pimecrolimus inhibits the release of mast cell mediators such as histamine [1, 18, 21]. In contrast to GC, the topical application of pimecrolimus does not affect the density of epidermal Langerhans cells [22], and does not alter the function of dendritic cells with respect to co-stimulatory molecule expression or T-cell proliferation [23]. In keratinocytes or endothelial cells, pimecrolimus does not affect cell adhesion molecule expression.
In contrast to pimecrolimus, tacrolimus modulates certain effects of inflammatory dendritic epidermal cells (IDECs) such as the expression of the high-affinity receptor for IgE (FcεRI) [21, 24, 25].
Tacrolimus also inhibits apoptosis of keratinocytes and T cells, thereby suppressing chemokine secretion by eosinophils and release of inflammatory mediators from mast cells [20]. In contrast to corticosteroids, neither tacrolimus nor pimecrolimus affects fibroblast functions such as collagen synthesis and therefore do not cause skin atrophy. In contrast to GC, pimecrolimus does not impair epidermal barrier function. This may explain why tachyphylaxis has not been observed upon treatment with topical CI.
In Vivo Effects
The in vivo antiinflammatory capacities of tacrolimus and pimecrolimus have been investigated in several animal models of contact dermatitis. Both compounds block the elicitation phase of contact dermatitis, thereby diminishing the inflammatory activity. The in vitro evidence of a significantly lower immunosuppressive potential of pimecrolimus in comparison to tacrolimus has been supported by in vivo animal studies. Here, pimecrolimus, in contrast to tacrolimus, had no effect on the sensitization phase of allergic contact dermatitis and thus apparently does not impair the primary immune response. This has been further supported by a variety of other animal models of immune-mediated diseases. Using a localized rat model of graft-versus-host reaction, pimecrolimus was significantly less effective than tacrolimus. In another rat model of kidney transplantation, pimecrolimus again was less effective in preventing graft rejection when compared to tacrolimus or cyclosporin A. Moreover, upon investigation of the effect on T-helper-cell–assisted B-cell activation in rats, pimecrolimus turned out to be significantly weaker when compared to that of tacrolimus [24, 26, 27].
Pharmacokinetic Studies
The question of potential systemic exposure is one of major concern in the development of a novel compound for topical application. The intact skin barrier restricts the passage of substances greater than 500 Da. As a result the penetration and permeation of topical cacineurin inhibitors whose molecular weight is greater than this (tacrolimus 822 Da, pimecrolimus 810 Da) is impaired. In addition, their lipophilic properties showed higher affinity for skin and lower potential for systemic absorption compared with other agents [125]. The capacity of pimecrolimus to penetrate into and permeate through the skin was investigated in vitro using human cadaver skin. This was compared to corticosteroids and tacrolimus. Accordingly, the amount of pimecrolimus penetrating into the skin was similar to that of corticosteroids or tacrolimus. However, pimecrolimus was observed to permeate significantly less through skin in comparison to corticosteroids or tacrolimus [28]. Therefore, one may suggest that following the topical application of pimecrolimus, the risk of systemic exposure is low and the ultimate possibility of systemic side effects is most unlikely [27]. This has been supported by several pharmacokinetic studies, which proved that after topical use of pimecrolimus in patients with atopic dermatitis, serum concentrations were equally low regardless of the age, severity of disease, and body area treated. In 99 % of the samples tested, concentrations were below 2 ng/mL, which is far below the level of 10–15 ng/mL, which is required for a systemic antiinflammatory effect [29].In contrast to tacrolimus, serum concentrations of pimecrolimus in this range did not cause systemic adverse events as has been shown in several clinical trials [30, 31].
Although the metabolism of pimecrolimus in the skin has not yet been carefully investigated, it might be assumed that it is removed by desquamation. In contrast, serum tacrolimus levels were detected more frequently following topical application in patients with atopic dermatitis. However, usually these levels were low and transient because circulating tacrolimus was no longer detectable upon improvement of skin barrier function after short-term treatment [32]. The reason for the observed differences between tacrolimus and pimecrolimus, however, is not completely understood. One possible explanation may be the different structure, lipophilicity, as well as content of lipophilic groups of these compounds. Moreover, pimecrolimus in contrast to tacrolimus has a high affinity for epithelial structures such as the skin but a low affinity for lymphoid organs [27].
Pharmacokinetic long-term studies over 1 year have further demonstrated [33] that the blood concentrations of pimecrolimus cream were rather low; moreover, no accumulation was observed and only a minimal increase was detected with increasing body surface area (BSA) during treatment [33, 34].
Clinical Studies on Efficacy and Safety of Calcineurin Inhibitors
Both CIs have been developed for the topical treatment of atopic dermatitis and are approved for this indication in many countries around the world. Tacrolimus can be obtained as 0.03 % and 0.1 % ointment (Protopic®), whereas pimecrolimus is available as a 1 % cream (Elidel®). While tacrolimus is approved for the treatment of moderate and severe atopic dermatitis in adults and children ≥2 years old, pimecrolimus cream (1 %) is available and approved for mild and moderate cases of atopic dermatitis in adults and children ≥2 years old. In some countries, pimecrolimus has been approved for the therapy of atopic dermatitis regardless of age and severity of the disease [37–39]. For both CIs, several clinical trials verified both compounds to be highly effective for the treatment of atopic dermatitis [9, 24, 40].
In January 2006 the US Food and Drug Administration (FDA) issued a boxed warning based on a theoretical risk of malignancy including lymphoma with topical CI use. In September 2010 the FDA released a review of TCI safety based on five peer reviewed studies. At that time they concluded that there may be a possibility of an association between tacrolimus and an increased risk of lymphoma. However epidemiological and clinical data since then have failed to demonstrate a causal link. Indeed in post marketing registries the observed number of malignancies and lymphomas has been very low and comparable or less than the number observed in the general population. In addition a number of published reviews have concluded that no conclusive proof has emerged to link TCI with malignancy in the 9 years since the boxed warning [131–133]. Meanwhile, topical CIs have been established or reported as alternative therapeutic strategies for skin diseases other than atopic dermatitis. Both tacrolimus ointment and pimecrolimus cream have been documented as successful treatment modalities for rosacea, lichen planus, psoriasis, lichen sclerosus et atrophicans, lupus erythematosus, and many others (reviewed elsewhere [39]).
From an economic point of view, in the long run CIs have been shown to be cost-effective [41], although at the moment they are more expensive than glucocorticoids [42]. However, as already mentioned, one has to consider age (infants, children, elderly people with skin atrophy), localization (face, groin, inframammary area), and severity (large proportions of the skin), for in these cases CIs are superior and safer therapeutic modalities. Thus, a fair calculation between CIs and other drugs such as glucocorticoids (GCs) may be difficult [41, 43, 44]. More studies are needed to further calculate costs of GC versus CI therapy.
In the first clinical trial of adults with atopic dermatitis, tacrolimus ointment significantly reduced skin lesions and pruritus within 3 weeks of treatment [45]. Subsequently, randomized double-blind controlled studies further defined the efficacy, tolerability, and safety of tacrolimus [44, 46]. In adults, 0.1 % tacrolimus ointment was as effective as hydrocortisone butyrate 0.1 % ointment [44, 47]. In children (2–15 years), 0.03 % ointment was more effective as compared to 1 % hydrocortisone acetate ointment [48], and more effective than a mild topical glucocorticoid ointment [44]. Subsequently, tacrolimus was compared to various topical glucocorticoids. In a meta-analysis of 25 randomized controlled trials, tacrolimus 0.1 % ointment was superior to hydrocortisone acetate (1 %), hydrocortisone valerate, and hydrocortisone butyrate (0.1 %), whereas tacrolimus (0.03 %) ointment was as effective as hydrocortisone acetate (1 %), but less effective than hydrocortisone butyrate.
After these successful short-term studies revealing efficacy of topical tacrolimus, a multi-center, open-label, noncomparative trial was performed [49]. Within the first week of treatment, most of these patients experienced a significant amelioration of eczema and pruritus. Of note, an increasing improvement was observed until month 3 after treatment. After 12 months, an excellent improvement (≥90 %) or clearance of the symptoms was reported in 68.2 % of patients. An improvement (≥50 %) was noted in 90.9 % of the cases [50]. Laboratory parameters did not change significantly during the study period. A burning sensation (47 %) usually terminated after initiation of treatment, and burning or itching was only occasionally reported [50]. Importantly, no tachyphylaxis was observed in these patients. The excellent long-term efficacy of tacrolimus ointment for the treatment of atopic dermatitis in children as well as adults was verified in several clinical studies [51]. By measuring tacrolimus plasma levels in patients treated with tacrolimus ointment it was revealed that in 67.1 % of patients tacrolimus plasma levels remained below the level of detection. High levels were found only in 0.4 % (≥5 ng/mL) of patients [5]. In patients with Netherton syndrome, blood concentrations over 20 ng/ml could be detected [53]. Despite these rare cases, systemic exposure after topical application of tacrolimus is very low. In summary, there is no evidence for systemic accumulation resulting in adverse side effects following the long-term treatment with tacrolimus ointment [54].
The efficacy of pimecrolimus 1 % cream for the treatment of atopic dermatitis in adults, children, and infants was verified in several clinical trials [4, 24, 39, 55–58]. Importantly, no significant drug-related adverse events were observed in these studies when applied twice daily. In comparison to both corticosteroids and tacrolimus, the capacity of pimecrolimus to permeate through the skin was significantly lower, indicating a very low risk of systemic exposure following the topical application of pimecrolimus cream [27]. This is also supported by studies with patients suffering from Netherton syndrome [59, 60]. In one recent study by Yan et al., three patients with Netherton syndrome received twice daily application of pimecrolimus 1 % cream over 18 months. In addition to the significant clinical improvements noted, blood levels recorded ranged from 0.625 to 7.08 ng/ml, revealing levels much lower than expected or required to cause systemic immunosuppression. These results were seen even when pimecrolimus was applied to 50 % of the body surface area [130] Therefore, long-term studies indicate a very low potential of systemic toxicity, immunosuppression, and local or systemic infections for pimecrolimus 1 % cream [61–63].
In children (2–17 years) and infants (3–23 months) with mild, moderate, or even severe atopic dermatitis, several multicenter clinical trials have further demonstrated [64] that pimecrolimus 1 % cream is highly effective within 8 days for treating both the eczema and the pruritus. No side effects including viral or bacterial infections were reported [56, 65, 66]. Thus pimecrolimus 1 % cream is a safe and effective therapeutic option in children and infants with atopic dermatitis. Of note, significantly more patients in the pimecrolimus group were maintained without glucocorticoid therapy [65, 67]. Due to its efficiency and low profile of adverse events, it is recommended to begin a topical CI therapy at an early stage of atopic dermatitis and probably for other inflammatory skin diseases [40, 68]. Under these circumstances, when the disease develops during CI therapy, intermittent use of other antiinflammatory compounds such as GC will be beneficial [69]. Tacrolimus ointment and pimecrolimus cream may even have a prophylactic effect when used intermittently after an episode of atopic dermatitis when patients still suffer from pruritus (every second to third day). In two clinical trials the efficiency of pimecrolimus was compared to that of glucocorticoids. In a short-term study pimecrolimus was less effective after 3 weeks than betamethasone valerate, although the maximal efficacy of pimecrolimus was not studied in detail. Moreover, in a long-term, double-blind, randomized multicenter clinical trial, the efficacy of pimecrolimus was compared to that of triamcinolone-acetonide 1 % cream or 1 % hydrocortisone. Although a significant improvement was observed in both groups, less severe side effects were observed in the pimecrolimus group [70–73].
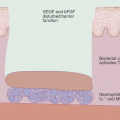
When comparing the effect of topical CIs with topical GC on the epidermal barrier it has been shown that both tacrolimus and pimecrolimus result in a significant increase in the number of lipid lamellae in the intracellular space. This increase is greater than that observed in the GC treated group. As the synthesis and storage of lipids in the lamellar bodies is essential for an effective barrier, this highlights an important therapeutic effect that TCIs have on repairing the epidermis and in reducing epidermal water loss [125, 126].This may suggest a superior effect of TCIs in repairing skin barrier architecture, preventing penetration of allergens and subsequent relapse.
Effects of Calcineurin Inhibitors on Innate Immunity and Host Defense
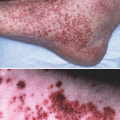
The safety and tolerability of tacrolimus ointment has been demonstrated in children and adults with atopic dermatitis. The most common local adverse event was a sensation of burning (in 29.9 % of children and 46.8 % of adults). Transient itching was noted in some children (23.1 %) and adults (25.8 %), which was most likely due not to infection but to neuronal activation [74]. The local adverse events were only noted during the first few days of treatment and were mild to moderate [51]. Concerning side effects on skin appendages, the risk of developing folliculitis or acne was increased in a few young adults [50].
An increased rate of bacterial skin infections could not be observed [50], and a decreased colonization with Staphylococcus aureus in the eczematous skin lesions was observed [75], which may be due to a normalization of cutaneous innate immunity after restoration of skin integrity. Atopic dermatitis patients exert an impaired capacity to produce antimicrobial peptides such as defensins and cathelicidins [76–82]. This effect may be due to a predominant TH2 immune response in atopic individuals. Moreover, IL-4 and IL-13, which are increased in atopic dermatitis, inhibit the production of antimicrobial peptides [82].
A slight, nonstatistically significant, increase of local viral infections such as herpes simplex was reported [11]. However, none of these cases caused a therapeutic problem [83]. The idea that pimecrolimus exerts long-term preventive effects in atopic dermatitis was verified by studies showing no relation between pimecrolimus treatment and the occurrence of eczema herpeticum [84, 85]. Moreover, clinical investigations verified that pimecrolimus 1 % cream was not associated with a significantly increased risk of the development of fungal, bacterial, or viral skin infections [61–63].
The effect of pimecrolimus on innate immunity in patients with AD was investigated in a double-blind, randomized, vehicle-controlled study which looked at its effect on IL-13 and antimicrobial peptides (AMPs) cathelicidin and human β defensin (HBD)-3. After 3 weeks of application a statistically significant reduction in IL-13 (which plays a pivotal role in the development of AD lesions) was observed when compared with the vehicle-treated group. In addition there was no significant reduction in catelicidin expression observed. While there was no increase in AMPs overall, its use was not associated with further suppression of the innate immune response [127]. This is in contrast to topical corticosteroids which have a more pronounced inhibition of AMP protein and mRNA levels suggesting a greater suppression of the innate immune system [126].
By using recall antigen tests, no impaired cellular immune response was observed in the skin even after long-term application of tacrolimus 0.1 % ointment [50]. Thus, tacrolimus-associated infections may not be regarded as a major risk factor in atopic dermatitis. Moreover, there is no evidence that the capacity to respond to vaccination with an appropriate antibody production is affected after topical pimecrolimus therapy. It does not alter the migratory capacity of antigen-presenting dendritic cells and does not impair the primary immune response. Despite these studies and reports, cessation of topical application with CI is recommended until total clearance of a viral infection. Surprisingly, the incidence of bacterial infections was found to be decreased during the application of pimecrolimus or tacrolimus. This was most likely due to a normalization of innate defense mechanisms [82].
Effects of Calcineurin Inhibitors on Pruritus
Calcineurin inhibitors play an important role in the treatment of atopic-induced pruritus [86]. Of note, a significant improvement of pruritus was observed within a few days of treatment using pimecrolimus cream [63], which has a beneficial effect on the quality of life in patients with atopic dermatitis [62]. Within 1 week of treatment pruritus was significantly decreased in these patients [87]. Thus, pimecrolimus 1 % cream is effective for the treatment of mild, moderate, and severe atopic dermatitis in adults as well as children and infants. In randomized multicenter, double-blind studies it was further demonstrated that significantly fewer infants treated with pimecrolimus developed severe flares as compared to controls [61, 63].
Itchy lesions that are often resistant to therapy, such as on the face and neck, also responded well to pimecrolimus therapy [65]. The same adverse events were also observed with tacrolimus in patients with atopic dermatitis, namely burning and a feeling of warmth. These sensations were regarded as mild and transient, lasting only 1–3 days [61–63]. This seems to be dependent on a transient release of preformed neuromediators such as substance P (SP) and calcitonin gene–related peptide (CGRP) from primary afferent nerve endings [74, 86, 88]. Siepmann et al. also demonstrated a significant improvement of pruritus in patients with prurigo nodularis (PN) when treated with topical pimecrolimus, including those patients with PN who did not have an atopic background. This randomized doubleblind phase II trial, demonstrated antipruritic effects within 10 days of starting treatment. It also demonstrated improvement of the prurigo nodules and dermatological quality of life [128].
Effects of Calcineurin Inhibitors on Atrophy
The effect of tacrolimus on fibroblast collagen formation was also determined in a double-blind study. Tacrolimus (0.03 % and 0.1 %), betamethasone valerate, and a vehicle control were compared after 1 week of application for skin thickness and procollagen peptide concentration in suction blister fluids. In contrast to betamethasone valerate, tacrolimus had no effect on procollagen propeptide production and caused no reduction of skin thickness [89]. Thus, the absence of skin atrophy is a major advantage in the treatment with CI [1, 7]. In summary, it is well documented that pimecrolimus does not affect collagen synthesis and therefore does not cause skin atrophy in mice or humans [90].
Risk of Pimecrolimus by Ultraviolet Exposure and in Skin Cancer
The use of systemic immunosuppressants such as cyclosporin A is well known to be associated with an increased risk for the development of ultraviolet (UV)-induced skin cancer such as basal cell carcinoma and squamous cell carcinoma, as well as of the development of actinic keratosis [91, 92]. The long-term experience with topical corticosteroids indicates that they might not be applicable for local treatment with immunomodulators. Therefore, it was necessary to analyze the incidence of developing skin cancer after treatment with tacrolimus. The incidence of skin cancer following the use of tacrolimus ointment has remained very low [93].
In contrast, it is well documented from animal studies that tacrolimus inhibits the development of phorbol ester-(TPA)-induced skin tumors [94].Tacrolimus also suppresses transforming growth factor-β1 receptor (TGFβ1R) activation [95], and prevents keratinocyte apoptosis [96]. Importantly, various animal studies have demonstrated that the topical application of pimecrolimus cream and additional UV-irradiation were not associated with an increased incidence of epidermal or melanocytic skin tumors [10]. Moreover, topical treatment with tacrolimus and pimecrolimus prevents the UV-mediated formation of dimethylthymidine dimers, suggesting a protective effect of these compounds against UV exposure [97]. However, future controlled studies are required to further elucidate the role of CI in UV-mediated skin damage. Meanwhile, a preventive strategy using appropriate sunscreens with topical CI treatment is recommended [10, 11, 98]. The question of tumor formation following long-term treatment with topical tacrolimus cannot be definitively answered at present. A recently published literature review looking at the extent to which topical CI use is associated with melanoma and non melanoma skin cancers found no evidence to date to support such an association. However limitations of the studies have meant that existing data to date is inadequate to give conclusive recommendations [129].Therefore, concomitant UV therapy should be avoided and the patients should be instructed to use UV-protective measures [10, 99].
Comparison of Topical Calcineurin Inhibitors
In a multicenter, randomized study, it was shown that tacrolimus 1 % ointment was more effective than pimecrolimus 1 % cream in adults and children with moderate/severe atopic dermatitis (AD) and at week 1 with mild AD. Tacrolimus was also superior with respect to itch scores and onset of action while no differences were observed concerning adverse side effects [100]. In summary, the first clinical trials already provided evidence for tacrolimus and pimecrolimus as safe and effective topical compounds for the treatment of AD in adults and children, with improvement both in pruritus as well as eczematous lesions [46]. A recent clinical investigator-blinded trial compared pimecrolimus 1 % cream and tacrolimus 0.03 % ointment in children. The efficacy of pimecrolimus 1 % cream was comparable to that of tacrolimus 0.03 % ointment. Pimecrolimus cream was better tolerated, and lesions in the face and neck healed faster after treatment with pimecrolimus cream [101–103]. However, one has to consider that the vehicle of tacrolimus and pimecrolimus is different: while tacrolimus is approved as an ointment, pimecrolimus is a cream. Therefore, patients with dry skin show a better tolerability of the ointment (tacrolimus), while the cream is predominantly preferred by patients with acute, erosive lesions.
Future Topical Anti Inflammatory Treatments
As a result of ongoing, successful research, exploring the pathophysiology of atopic dermatitis and its inflammatory pathways, potential therapeutic targets have been identified. This has resulted in the development of some novel topical anti inflammatory agents, many of which are currently in clinical trials.
Phosphodiesterase inhibitors (PDEs) inhibit the degradation of cyclic adenosine monophosphate (cAMP) and have emerged as potential topical treatments for AD. Blocking PDE4 reduces TNF-α gene expression in macrophages and dendritic cells and also reduces T cell proliferation and production of proinflammatory cytokines IL-2, IL-4 and IL5. They also enhance the expression of IL-10 [135]. The topical application of E6005, a novel PDE4 inhibitor has been demonstrated to have significant antipruritic activity in mice with chronic atopic dermatitis [136]. As a result a phase 1 study looking at the therapeutic action of a topical PDE4 inhibitor is currently underway in patients with atopic dermatitis.
NF-kB decoy is a double-stranded deoxyribonucleic acid (DNA) oligodeoxynucleotide that mimics the NF-kB binding sequence on chromosomal DNA, thereby inhibiting the production of the inflammatory response triggered by NF-kB. The efficacy of topical NF-kB decoy in AD has been demonstrated in a number of mouse models [136, 137]. It reduces the expression of inflammatory cytokines such as IL-1β, TNF-α, ICAM-1 and macrophage inflammatory protein 2-α precurser, thereby reducing inflammation and restoring skin barrier function [137]. A phase 2 study was subsequently performed to evaluate the safety and tolerability of twice daily application of NF-kB decoy to adults and results are pending.
WBI-1001 is a novel synthetic compound demonstrating nonsteroidal antiinflammatory activity. It was originally derived from metabolites of a unique group of bacterial symbionts of entomopathogenic nematodes. It has been shown to inhibit proinflammatory cytokines including IL-2, IL-13, IL-17A and TNF-α [140]. Bissonnette et al., in a 12 week, multicenter, randomized, placebo-controlled double-blind trial demonstrated that topical WBI-1001 at concentrations of 0.5 % and 1.0 % was an efficacious and safe topical treatment for patients with mild to severe AD. However the study did not match the effectiveness to an active comparator and so further studies are required [141].
Mapracorat is a selective glucocorticoid receptor agonist (SERGA) which is currently in two phase 2 clinical trials evaluating its safety and efficacy in patients with AD. It is a highly selective glucocorticoid receptor ligand with immunomodulatory and antiinflammatory effects but with a more favourable side effect profile than topical GCs [139]. Its effect has been demonstrated at a cell signalling level by the inhibition of p38 mitogen-activated protein kinase (MAPK), C-Jun N-terminal kinase (c-JNK), activator protein 1 (AP-1) and NK-kB transcriptional activity.
Conclusion
Topical CIs have established a broad, effective, and safe treatment modality for mild to severe subtypes of atopic dermatitis and other inflammatory skin diseases. During the usage of topical tacrolimus or pimecrolimus, respectively, adverse side effects are rare. Topical CIs are the first antiinflammatory compounds that are suitable for effective, long-term treatment of inflammatory skin diseases. Moreover, they also may be used as an early local therapy when the first signs of itching and eczema appear. Perhaps early and effective local therapy using these novel compounds in infants and children may even have a preventive effect [5, 11]. Therefore, early therapeutic intervention is recommended when lesions or pruritus occur. However, clinical studies are still required to investigate the course of the chronic skin disease treated with CIs with respect to the frequency and severity of the skin lesion. There is evidence that the quality of life in these patients and their relatives has significantly improved [5, 11].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree