Fig. 7.1
Clinical picture of melasma where biopsy was performed (a) and histopathology (b) showing larger melanocytes in the epidermis accompanied by solar elastosis, sparse lymphocytic infiltrate, and few pigment-laden macrophages in the dermis (H&E, ×400)
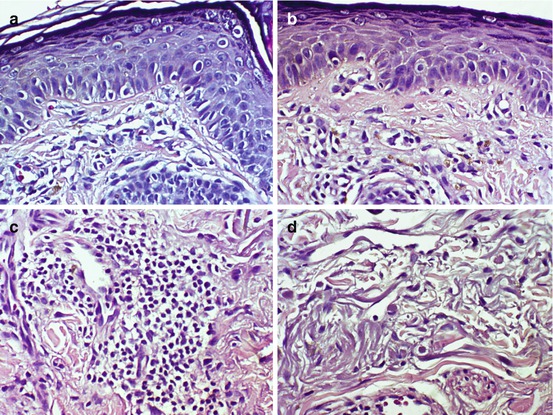
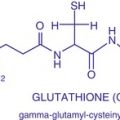
Fig. 7.2
Histopathological findings of melasma characterized by (a) large melanocytes in the epidermis, (b) pigment-laden macrophages in the papillary dermis, (c) perivascular lymphocytic infiltrate, and (d) solar elastosis (H&E ×400)
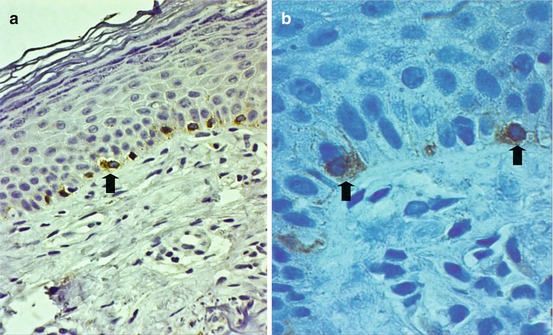
Fig. 7.3
Immunohistochemical marker Melan-A highlights larger, intensely staining melanocytes (arrow) in the basal cell layer (H&E ×400 (a), H&E ×1000 (b))
Electron microscopy revealed more melanosomes in keratinocytes, melanocytes, and dendrites in the involved skin, in comparison to the uninvolved skin. Miot et al. [10] demonstrated similar findings of greater epidermal melanin in the epidermis. In addition, dermal findings include a mild lymphohistiocytic infiltrate and solar elastosis in lesional melasma skin. A mild lymphohistiocytic infiltrate in 75 % of hyperpigmented areas was likewise observed by Grimes et al. [8]. Pigment-laden macrophages were present both in melasma lesional and perilesional normal skin in 36 % of Korean patients and in all 11 melasma patients of Fitzpatrick skin types IV to VI [8, 9]. The degrees of basement membrane damage and vascularization have not been extensively studied and warrant further investigation.
Presently, few conclusions can be drawn from evaluating all light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma: (1) that melasma is a consequence of specific hyperfunctional melanocytes that cause excessive melanin deposition in the epidermis and dermis and (2) that melasma is characterized by epidermal hyperpigmentation with or without melanophages and raising the question if there is indeed a “dermal variant” [7–9].
7.2 Histopathology of Ochronosis
Two cases of hydroquinone-induced ochronosis in two Filipinos with Fitzpatrick skin type IV showed on histology numerous fragmented reddish-brown fibers in various configurations, some of which appear crescentic or “banana shaped.” Swelling and homogenization of collagen bundles are seen in the papillary and reticular dermis. The blood vessels are telangiectatic and mild solar elastosis is seen (Fig. 7.4) [11, 12].

Fig. 7.4
Hydroquinone-induced ochronosis in a Filipino (a) and histopathological findings (b) of yellowish-brown-colored material in the dermis accompanied by solar elastosis, mild lymphocytic infiltrate, and pigment-laden macrophages (H&E ×400)
Early lesions show basophilic and swollen collagen fibers before developing the characteristic yellow ochronotic morphology. Fully developed lesions are characterized by focal collections of refringent, yellow-brown, sharply defined, irregularly shaped, and frequently fragmented fibers in the superficial dermis. There is often coexisting mild solar elastosis [13, 14]. Depigmentation of epidermal melanocytes is usually associated with pigment-laden macrophages in the papillary dermis [15]. Pigment granules are often present in the epithelium and basement membrane of sweat glands, in endothelial cells, and within dermal macrophages [16]. In chronic lesions, large amorphous eosinophilic granules may develop, resembling colloid milium [17]. An infiltrate of histiocytes is present focally in relation to some of the deposits [14]. The granulomatous reaction is believed to contain and selectively destroy the fragments of ochronotic fibers. Occasionally ochronotic material can be identified in giant cells [18].
Electron microscopic studies have shown electron-dense ochronotic bodies embedded in a granular material infiltrating the adjacent collagen bundles. The swollen collagen fibrils characteristically lose their banding pattern, subsequently degenerate, and are replaced by amorphous ochronotic pigments. The fibrils rupture and the pigments scatter free in the dermis. These pigments are phagocytosed later on by macrophages and giant cells [18–20].
7.3 Histopathology of Dermal Melanocytosis (Acquired Bilateral Nevus of Ota-Like Macules (ABNOM) or Hori’s Macules, Nevus of Ota, and Sun’s Nevus)
Dermal melanocytoses comprise a variety of congenital and acquired conditions characterized by a sparse population of intradermal dendritic, variably pigmented, spindle-shaped melanocytes, with or without the presence of dermal melanophages. These forms of facial melanoses are most commonly found in the skin of Asians and other darkly pigmented people. Nevus of Ota, acquired bilateral nevus of Ota-like macules (ABNOM), and acquired unilateral nevus of Ota, also known as Sun’s nevus, represent distinct types of dermal melanocytosis occurring on the face [21, 22].
ABNOM shares similarities to melasma with regard to clinical features, including female preponderance, acquired onset, and principal involvement of the malar area [23]. However, the histopathological elements are distinct. Controversy still exists whether what was previously thought of as dermal melasma is actually ABNOM or other forms of dermal melanocytoses.
Lee et al. [24] compared the histopathological characteristics of ABNOM with nevus of Ota using hematoxylin and eosin, GP-100 (NK1-beteb), and Fontana-Masson stains. In the epidermis, there was no difference in the density of melanocytes and pigments between ABNOM and nevus of Ota. In the dermis, the differences between ABNOM and nevus of Ota have been documented. The spindle-shaped melanocytes in ABNOM were concentrated perivascularly in the superficial dermis. In contradistinction, the spindle-shaped melanocytes in nevus of Ota were more numerous and distributed interstitially in both the superficial and deep dermis. The long axis of these melanocytes was oriented parallel to the collagen bundles (Fig. 7.5). Lee et al. [23] also demonstrated that solar elastosis is slightly more prominent in the lesional skin of ABNOM. The increased dermal expression of stem cell factor (SCF) and c-kit in ABNOM may suggest a possible role in the pathogenesis of this pigmentary disorder.
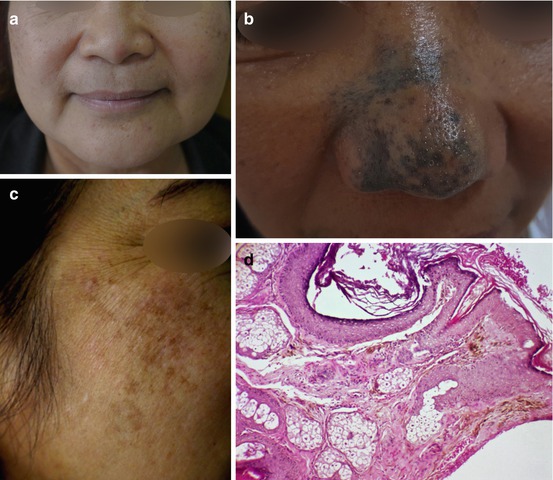

Fig. 7.5
Clinical photos of ABNOM (a, b), acquired nevus of Ota/dermal melanocytosis, (c) and histopathological findings (d) of heavily pigmented, spindle-shaped melanocytes in the dermis (H&E ×100)
The degree of melanin pigmentation and number and distribution of melanocytes per unit area in the epidermis and dermis were not significantly different in ABNOM as compared to extrafacial forms of acquired dermal melanocytosis suggesting that both diseases form part of the same disease spectrum [25].
7.4 Histopathology of Ashy Dermatosis, Erythema Dyschromicum Perstans, and Lichen Planus Pigmentosus
Ashy dermatosis (AD), erythema dyschromicum perstans (EDP), and lichen planus pigmentosus (LPP) present as acquired macules and patches of hyperpigmentation. The etiology and pathogenesis of each disease remains an enigma; thus, there is no effective widely accepted treatment. The clinical features overlap and racial variations may exist. The diseases share common histological features but subtle differences have been noted.
Salient histological features of ashy dermatosis from the original study of Ramirez [26, 27] were liquefaction degeneration of the basal layer with a perivascular infiltrate of histiocytes, some lymphocytes, and melanin-laden macrophages (Fig. 7.6). A lichenoid tissue reaction pattern was likewise observed in “erythema dyschromicum perstans” when the disease was introduced by Convit et al. in 1961 [28].
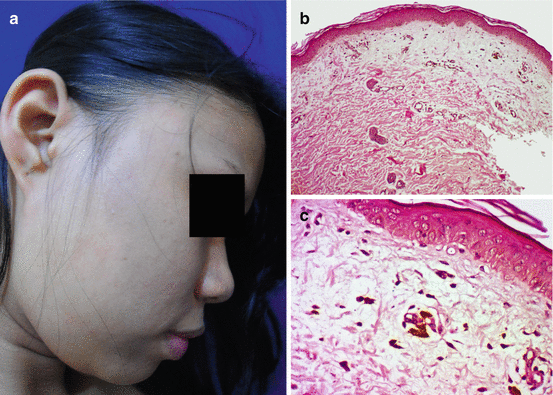

Fig. 7.6
Ashy dermatosis in a Filipino (a) and histopathological findings (b, c) of subtle vacuolar change in the basal layer and prominent pigment incontinence in the dermis (H&E ×100 (b), ×400 (c))
Bhutani et al. [29] reported 40 cases in India with lesions similar to those described by Ramirez. However, clinical and histological findings in one-third of patients had association with lichen planus; they named them lichen planus pigmentosus. It is believed to be a macular variant of lichen planus and more recent observations show that it usually starts on the face, neck, and earlobes. It is usually associated with pruritus and oral mucosal involvement is seen [30]. The histopathological findings have similarities to lichen planus (Fig. 7.7).
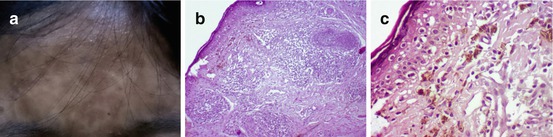

Fig. 7.7
Lichen planus pigmentosus in a Filipino (a) and histopathological findings of (b) a lichenoid and periadnexal dermal infiltrate (H&E ×100), (c) prominent vacuolar alteration of the basal layer with Civatte bodies, and numerous pigment-laden macrophages (H & E ×400)
Controversy exists regarding the relationship of AD, EDP, and LPP based on clinical and histopathological features. Pinkus [31, 32] included ashy dermatosis and atrophic lichen planus in the same section of his classification of “lichenoid tissue patterns” because of their histologic similarities. Novick and Phelps [33] suggested that EDP is a variant of lichen planus. On the other hand, Vega et al. [34] reviewed 20 cases of AD and 11 cases of LPP in 1992. They distinguished these two entities despite similar histopathologic findings and believed that ashy dermatosis predominates in type IV skin, and there are probably some undefined ecological and nutritional conditions that are not applicable to other races or countries in North America and Europe.
To differentiate AD from EDP, Weedon [35] recently proposed that the term EDP should be used in cases where lesions have or have previously had an erythematous border, while AD should be used for other cases where the “erythematous feature” is lacking. Histologically, EDP shows a patchy vacuolar alteration of the epidermis, pigment incontinence, and moderately dense lymphocytic infiltrate of lymphocytes. In contrast, AD reveals atrophy of the epidermis with subtle vacuolar alteration of the basal cell layer, marked melanin incontinence, and mild infiltrate referred to as “burnt-out” appearance [35]. The “burnt-out” appearance is commonly used to describe post-inflammatory pigmentary alteration following interface dermatitis such as in lichen planus, lupus erythematosus, erythema multiforme, and lichenoid drug eruptions.
The erythematous component of EDP demonstrates a vacuolar alteration of the basal cell layer, the presence of occasional colloid bodies, pigment-laden macrophages, and a perivascular infiltrate of lymphocytes and histiocytes. In the hyperpigmented macules and patches, pigment incontinence is a more prominent histological finding, while the vacuolar change in the basal cell layer and the lymphohistiocytic infiltrate in the dermis may only be mild or absent [36].
The histopathological findings of LPP is characterized by an atrophic epidermis with vacuolar alteration of the basal layer; a scarce lymphohistiocytic or lichenoid infiltrate with incontinence of pigment and the presence of melanophages are seen in the dermis [34].
For LPP, a biopsy of a raised lesion shows typical lichen planus pathology characterized by saw toothing of the rete ridges, wedge-shaped hypergranulosis, vacuolar alteration of the basal layer, Civatte bodies, and lichenoid lymphocytic infiltrate. A biopsy of a macule, on the other hand, reveals a relatively flat epidermis with loss of the rete pattern, focal vacuolar change with occasional necrotic keratinocytes and Civatte bodies, dermal melanophages, and lymphocytic infiltrate [33]. The lichenoid inflammatory infiltrate, which is often focal and peri-infundibular, is a helpful histological feature which may differentiate LPP from either EDP or AD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree