The gracilis free flap is the ideal modality of emotive and spontaneous facial reanimation in patients with a viable contralateral facial nerve. A 2-stage procedure with a cross-face nerve graft followed by gracilis free flap inset is advocated. In this article, the anatomy of the gracilis muscle, alternative neural sources (including the masseteric nerve), and technical aspects of the procedure are discussed. The literature regarding outcomes and complications is reviewed.
Key points
- •
The neurovascular pedicle of the gracilis free flap is composed of a single arterial branch arising most commonly from the adductor branch of the profunda femoris, two vena comitantes, and the obturator nerve.
- •
Numerous neural sources have been used in combination with the gracilis flap, including the masseteric, hypoglossal, spinal accessory, and phrenic nerves. A cross-face nerve graft that uses a functioning contralateral facial nerve branch allows for the greatest degree of emotive and spontaneous reanimation.
- •
A 2-stage procedure involving a cross-face nerve graft in stage 1 and, after axonal regeneration has occurred, a gracilis free flap in stage 2, has been found to be highly successful in dynamic reanimation.
- •
In patients with paralysis of less than 2 years duration, cranial nerve substitution should be considered in stage 1 to decrease atrophy of the facial musculature.
- •
Outcomes using the described 2-stage procedure are excellent, with a low flap failure rate and significant improvement in facial symmetry/smile excursion; however, it is not uncommon for patients to require a third stage to refine the flap.
Introduction
Facial paralysis is a devastating condition that often results in significant negative social and psychological outcomes. Demonstrating the social value on facial expression, Sinno and colleagues found that healthy individuals would be willing to sacrifice 8 years of life and undergo a surgery associated with 21% mortality to correct a facial paralysis. Certainly, optimal recovery of facial function can be achieved when immediate neurorrhaphy of a freshly transected nerve is performed; however, this is often not feasible due to the etiology of paralysis. Ideal treatment of a unilateral facial paralysis not amenable to direct repair would restore resting tone/bulk and would allow for symmetric, spontaneous, and dynamic function. Smile, although a very important aspect of facial expression, is not the only endpoint in rehabilitation. Normal social interaction relies on our ability to react to conversation with subtle facial expression changes. In addition to smile, re-creation of such subtle facial movements is the ultimate goal in facial reanimation.
Free-tissue transfer has afforded a modality of restoring bulk, dynamic function, and spontaneity, with the gracilis free flap presently leading as the gold-standard donor muscle. In this article, we focus on the gracilis free flap as it is used in dynamic facial reanimation. We discuss a 2-staged treatment used by the senior author (BA) that achieves optimal restoration of resting tone and spontaneous movement.
Introduction
Facial paralysis is a devastating condition that often results in significant negative social and psychological outcomes. Demonstrating the social value on facial expression, Sinno and colleagues found that healthy individuals would be willing to sacrifice 8 years of life and undergo a surgery associated with 21% mortality to correct a facial paralysis. Certainly, optimal recovery of facial function can be achieved when immediate neurorrhaphy of a freshly transected nerve is performed; however, this is often not feasible due to the etiology of paralysis. Ideal treatment of a unilateral facial paralysis not amenable to direct repair would restore resting tone/bulk and would allow for symmetric, spontaneous, and dynamic function. Smile, although a very important aspect of facial expression, is not the only endpoint in rehabilitation. Normal social interaction relies on our ability to react to conversation with subtle facial expression changes. In addition to smile, re-creation of such subtle facial movements is the ultimate goal in facial reanimation.
Free-tissue transfer has afforded a modality of restoring bulk, dynamic function, and spontaneity, with the gracilis free flap presently leading as the gold-standard donor muscle. In this article, we focus on the gracilis free flap as it is used in dynamic facial reanimation. We discuss a 2-staged treatment used by the senior author (BA) that achieves optimal restoration of resting tone and spontaneous movement.
History
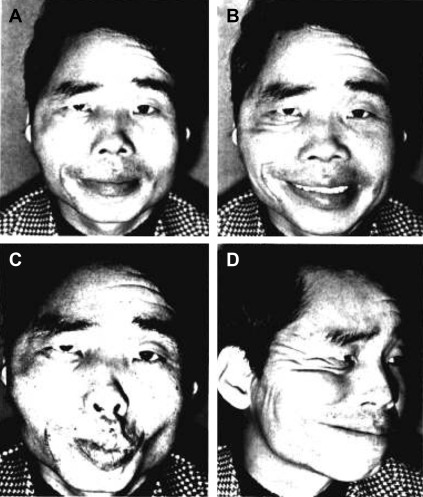
Following the advent of free-tissue transfer, the gracilis muscle was identified as a useful source of tissue for reconstruction of small traumatic or iatrogenic defects. Its utility was based on its relative accessory function in leg adduction, consistent anatomy, and comparative ease of harvest. Transplantation of the gracilis muscle was first described in 1952 by Pickrell and colleagues, who used the muscle for rectal sphincter reconstruction. In the 1970s, Harii and colleagues were the first to use the gracilis free flap in the head and neck, using it to reconstruct traumatic and iatrogenic temporal defects with successful outcomes. Harii and colleagues subsequently went on to use the flap for dynamic reanimation of facial paralysis, using the deep temporal nerve for neurotization. This method of reinnervation allowed for smile via a voluntary bite action; however, the results appeared overexaggerated and the smile was not, by design, spontaneous ( Fig. 1 ).
O’Brien later used a 2-stage, cross-facial nerve graft (CFNG) technique with a gracilis free flap. The cross-facial approach was based on the method pioneered by Thompson and Gustavoson, in which a CFNG was sutured to the distal buccal branches of the unaffected facial nerve and anastomosed with the motor nerve of an extensor digitorum brevis free flap on the paralyzed side. Using the gracilis flap and a CFNG, O’Brien and colleagues found a 51% success rate in a cohort of 62 patients. Although their early work demonstrated mixed results, their technique established a starting point in dynamic reanimation using the gracilis flap.
Alternate donor sites
In the late 1980s, interest in dynamic facial reanimation grew, and a number of other donor sites for free-muscle transfer were explored. The pectoralis minor, serratus anterior, latissimus dorsi, extensor digitorum longus, rectus femoris, and rectus abdominis were all studied as potential donor muscles in dynamic facial reanimation. Of these, the gracilis, pectoralis minor, and latissimus dorsi have demonstrated the greatest success.
Harrison and Grobbelaar published the results of 637 patients treated for unilateral facial paralysis with pectoralis minor free-muscle transfers between 1981 and 2008. Their experience found that 354 had an excellent outcome, as assessed by the operating surgeon; 27.2% required revision procedures, and 13.3% had delayed tightening of the muscle.
The latissimus dorsi has also been demonstrated as a fair candidate for dynamic facial reanimation. Takushima and colleagues published results of their 1-stage latissimus dorsi muscle transfer, whose success may approach that of the gracilis flap. In this report, 87% of cases demonstrated muscle contraction in analysis of 351 transfers. Their group also noted that the latissimus dorsi provides the optional use of an overlying skin paddle, which can be beneficial in reanimation after ablative procedures. The bulk of this flap, however, does limit its utility in non-ablative reanimation.
Despite the plethora of described donor sites, new candidates for free-muscle transfer are still under investigation. Alam and colleagues recently published preclinical results of cadaveric evaluation of the sternohyoid muscle. Their findings demonstrated that the superior thyroid artery is reliable as the vascular pedicle for this novel flap. Given the length of the motor nerve (the ansa cervicalis), which could allow for a single-stage procedure, the thin muscle caliber, and nonessential endogenous function, this muscle could represent an excellent candidate for reanimation in the future, pending further clinical evaluation.
At the present time, the gracilis free flap is the gold standard for free-tissue facial reanimation. Its thin bulk, contractile strength, reliable pedicle anatomy, and low donor-site morbidity make it an ideal donor site in dynamic reanimation.
Anatomy
The gracilis muscle is one of the adductors of the leg, located in the medial thigh. It is the most superficial of all of the medial thigh muscles. It originates from the ischiopubic ramus and inserts onto the medial tibia below the condyle via the pes anserinus. On average, the muscle measures approximately 25 cm in length. Owing to its name, based on the Latin word gracile, meaning slender, it is straplike and thin, tapering from superior to inferior from approximately 6 cm to 4 cm. At its insertion, it is located posteromedial to the adductor longus ( Fig. 2 ). This muscle and its tendinous insertion are readily palpable, thereby facilitating identification of the gracilis muscle.
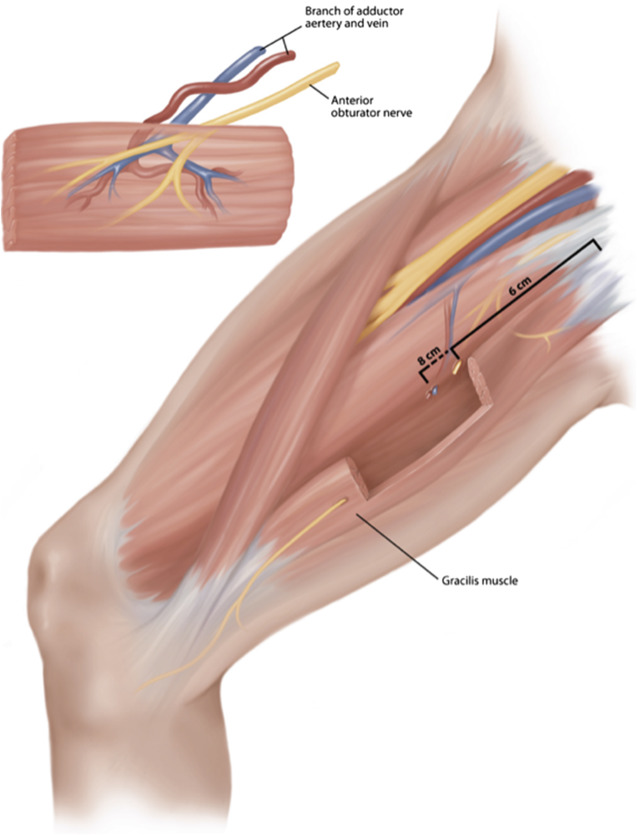
A single arterial branch arising most commonly from the adductor branch of the profunda femoris comprises the arterial supply to the proximal muscle in 73% to 87% of patients. The vessel is on average 1.6 mm in diameter. Infrequently, the arterial supply can alternatively be found to arise from the medial circumflex artery or combination of both of these arteries. The vascular pedicle is found entering the undersurface of the proximal third of the muscle after passing between the adductor longus and brevis. Its entry point is typically 8 to 10 cm below the pubic tubercle. Two vena comitantes draining into the deep leg venous system can be found running with the artery.
The muscle is innervated by the anterior branch of the obturator nerve, which can measure up to 12 cm in length. It is typically found approximately 6 cm from the pubic tubercle. The motor nerve often divides into branches innervating the superior and inferior muscle separately. This division can be used for division of 2 functioning units.
Nerve selection for neurotization
Beginning with Harii and his use of the deep temporal branch of the mandibular division of the trigeminal nerve, a number of modalities for neural power in free-tissue transfer have been described. Selection of the appropriate nerve for innervation of the gracilis free-tissue transfer hinges on patient goals and the etiology of paralysis.
In the 1970s, the CFNG was developed by Smith and Scaramella, using an intact contralateral facial nerve for innervation of the paralyzed side. The goal of such a technique was to attain synchronized and spontaneous movement in the case of unilateral paralysis. Initially, this methodology was used in an effort to neurotize the endogenous paretic muscles, but was found to be limited in its success should more than a few months elapse between onset of paralysis and neurotization. Although its use in this regard may have been limited, it was later applied to free-tissue transfer, initially in a 1-stage procedure in which the CFNG was placed in the same procedure as the free-tissue transfer. The process was later modified to a 2-stage procedure, which is most commonly used today.
Compared with other neural sources, the use of the CFNG in combination with a gracilis free flap allows for the greatest degree of spontaneity in facial expression, and is the preferred methodology for neurotization by the senior author (BA). The senior author (BA) uses a 2-stage procedure. In the first stage, a sural nerve graft is tunneled from the zygomatic branch of the intact facial nerve to the contralateral gingivobuccal sulcus. Six to 9 months is allowed for axonal regrowth, and thereafter, the nerve graft is anastomosed to the obturator nerve of the gracilis in the second stage. Technical aspects of the procedure are described in more detail later in this article.
In recent years, a single-stage procedure wherein the obturator nerve is directly tunneled across the upper lip and connected to the contralateral facial nerve has been studied. Although it does have the benefit of the decreased morbidity of a second procedure, and similar to the 2-stage procedure, allows for spontaneous facial expression, it was found to have a decreased degree of symmetry at rest, and thus, is thought to have a less favorable result.
Of course, not all patients have an available contralateral facial nerve, as is the case in Mobius syndrome (congenital absence of bilateral facial nerves). In such patients, a CFNG is not a viable option. The nerve to the masseter is the most commonly used source of reinnervation in such patients. As compared with the CFNG, this neural source does not allow for the same degree of spontaneity, given that generation of smile depends on a conscious bite action; however, it has been found to allow for a greater degree of excursion of the oral commissure compared with the CFNG. Additionally, there are studies that report that most patients are able to develop some degree of spontaneous smile over time due to cortical plasticity. Dual innervation with CFNG and masseteric nerve is being currently studied at several centers.
Other neural sources, such as the phrenic, spinal accessory, and hypoglossal nerve, have also been attempted. These nerves are not commonly used given the high donor site morbidity (hemitongue atrophy/deviation in the case of the hypoglossal and trapezius atrophy in the case of the spinal accessory nerve). Moreover, significant voluntary movement is required to generate facial expression with the use of these neural sources.
Patient selection
Numerous traumatic, malignant, congenital, iatrogenic, and infectious etiologies can result in facial paralysis. A clear delineation of mechanism of paralysis is critical in development of a treatment plan. Traumatic or iatrogenic facial nerve injury to the distal branches of the facial nerve may be amenable to a direct end-to-end anastomosis if identified quickly, and this approach affords the best outcome. Ideally, direct repair would take place within 3 days, before axon degeneration, which allows for the use of a nerve stimulator for identification of the distal branches, but in general, the longer the delay in repair, the worse the outcome on facial function.
Duration of symptoms is also critical, as patients with paralysis from Bell Palsy may continue to improve as much as a year or more from onset. Before this time, such patients should not be offered reanimation. Patients with paralysis related to a parotid or temporal bone malignancy with large surgical defects and/or a history of radiation therapy to the area are not typically offered CFNG/free muscle transfer due to limited outcomes in the setting of a previously operated/irradiated surgical field. These patients are advised to undergo orthodromic temporalis tendon transfer by the senior author (BA).
In cases of complete paralysis not amenable to direct repair or otherwise thought to be unlikely to recover, the patient should be considered a candidate for reanimation. The senior author proposes a treatment plan based on classification of patients into 1 of 4 groups according to etiology and duration of paralysis (summarized in Table 1 ).
| Severe/Complete paralysis <2 y | Two-stage cross-facial nerve graft (CFNG)/gracilis free muscle transfer |
| Nerve substitution procedure at first stage | |
| Severe/Complete paralysis >2 y | Two-stage CFNG/gracilis free muscle transfer |
| Partial paralysis with Synkinesis | Selective neurectomy of the lower division facial nerve; possible 2-stage CFNG/gracilis free muscle transfer |
| Bilateral paralysis | One-stage masseteric nerve to gracilis free muscle transfer or Bilateral orthodromic temporalis tendon transfer |
Severe/Complete Paralysis Less Than 2 Years
Individuals who develop acute paralysis secondary to intracranial tumors or temporal bone fracture, in which the proximal branch of the facial nerve is not accessible, are amenable to cranial nerve substitution techniques with the coaptation of the distal trunk of the facial nerve to the hypoglossal or masseteric nerves. It is critical that the facial nerve injury is deemed permanent and complete before embarking on these procedures, as they require transection of the facial nerve distal to the stylomastoid foramen. Furthermore, it is crucial that the procedure is undertaken less than 2 years after the facial nerve paralysis to ensure that the facial musculatures have not developed permanent atrophy. Cranial nerve substitution techniques do an excellent job of restoring tone and providing dynamic reanimation. The smile mechanism, however, is not spontaneous, requiring extensive retraining and can also result in significant synkinesis. The senior author often combines cranial nerve substitution techniques with CFNG and secondary gracilis flap to improve resting tone and restore spontaneous smile mechanism.
Severe/Complete Paralysis Greater Than 2 Years
Long-standing unilateral paralysis has also been observed to have success with CFNG and second-stage gracilis flap; however, due to the duration of paralysis and long-term atrophy, these patients are unlikely to improve bulk/tone with an additional cranial nerve substitution procedure.
Partial Paralysis with Synkinesis
Patients with partial paralysis often suffer from synkinesis, in which the simultaneous contraction of the facial muscles can result in activation of contradictory muscles, thereby causing an “auto-paralysis,” in which patients may be unable to generate a meaningful smile. These patients are unlikely to benefit from an additional nerve substitution (ie, XII-VII procedure), as they have not experienced denervation and atrophy of the facial muscles. In the senior author’s practice, such patients are treated with botulinum toxin, neuromuscular retraining, and selective neurectomy of the lower division of the facial nerve. Some severely affected individuals may also benefit from a 2-stage CFNG with gracilis free tissue transfer.
Bilateral Paralysis
Bilateral facial nerve paralysis is rare, seen in only 1.5% of affected patients. Etiology is most commonly congenital, autoimmune, or infectious. In patients who do not have a functional contralateral facial nerve, as is the case in infectious bilateral paralysis or Mobius syndrome, among others, there are no functional branches of the facial nerve to use for CFNG. Therefore, reanimation in these patients is carried out in a single-single stage procedure using the masseteric branch of mandibular nerve. The result is less natural, requiring the patient to bite down to generate a smile. There is no spontaneity to facial expression, as all movement must be voluntarily generated. This article focuses on CFNG using the zygomatic branch of the facial nerve in cases of unilateral paralysis. For further details regarding masseteric nerve innervation in free tissue transfer, refer to publications by Manktelow and colleagues.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






