Introduction
As we further elucidate the molecular and genetic basis of scars and wound healing, researchers are able to develop more targeted therapies and minimally invasive treatment modalities. This chapter delves into novel therapeutics and ongoing clinical trials for scar management. We will also discuss the psychology of scars, their impact on body image, and the role of interdisciplinary collaboration for scar patients.
Future Directions: Novel Therapeutics
Micro RNA
Micro RNAs (miRNA) are small noncoding RNA sequences that can modify gene expression to ultimately impact protein synthesis. The function of miRNAs in the body is complex, as the role of these molecules varies by organ system and cell type. In medicine, miRNAs are being explored as a potential therapeutic avenue to decrease expression of pathologic genes that play a role in various illnesses—including neurological conditions, malignancies, and viral infections—or to enhance response to a treatment by targeting drug resistance. A growing body of evidence suggests the pathophysiologic role of miRNA expression for hypertrophic and keloid scar formation and wound healing.
Various miRNA subtypes, including miR-21 and miR-23b-3p, play a role in keloid formation by increasing fibroblast expression. In 2022, Kuai and Jian showed inhibiting miR-23b-3p upregulation suppressed expression of collagen and fibronectin in keloid tissues. This strand of miRNA normally inhibits the regulatory ubiquitin-editing enzyme A20, ultimately allowing for scar hyperproliferation. Similarly, miRNA-31 is overexpressed in keloid tissue leading to excess collagen deposition via the vascular endothelial growth factor (VEGF) pathway. Further work is needed to develop therapeutics that disrupt these sequences and ultimately allow for controlled wound healing. Inhibitory miRNAs are also present in the scar formation pathway and can potentially be upregulated to deter hypertrophic scar formation. For instance, miR-7846-3p derived from adipose stem cells decreased angiogenesis and proliferation of keloid fibroblasts. Additionally, miRNA-182-5p was shown to act via the SMAD4 pathway to decrease fibroblast proliferation on an in vivo rabbit ear model.
Several methods are being explored for miRNA therapeutics, including delivery of recombinant synthetic miRNA strands directly into target tissue, versus administration of small proteins that can enhance or inhibit miRNA function as appropriate. For patient administration, trials are assessing direct injection techniques along with transdermal or biodegradable patches as discussed later in this chapter.
Small Interfering RNA
Similar to miRNA, small interfering RNA (siRNA) are short noncoding RNA sequences that play a role in gene silencing and can inhibit pathologic processes. In 2022, Chun and colleagues investigated siRNA-mediated silencing of secreted protein acidic and cysteine-rich (SPARC), a sequence that upregulates collagen production resulting in hypertrophic scars. Results of this in vitro study showed significantly reduced SPARC gene expression without notable cytotoxic effects. Although these preliminary results are promising, further human clinical trials are needed to fully assess safety and efficacy.
Drug Delivery Methods
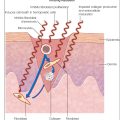
Although device-assisted drug delivery was discussed earlier in this text as a therapeutic strategy for scar management, microneedle patches are also an emerging option for transdermal drug delivery. These patches are formulated with durable and minimally invasive microneedles that can be coated with various medications, allowing extended release of the drug with less pain than a traditional intradermal injection. This method may also allow for self-administration and avoid the time and costs associated with a clinic visit for provider administration of an injectable. In 2023, Disphanurat and colleagues assessed efficacy of a triamcinolone acetonide–coated microneedle patch (loaded with 0.96 mg triamcinolone per patch, with one patch applied per centimeter of scar length) versus placebo in a split scar study. Although intralesional injection with corticosteroids +/− 5-fluorouracil is the current standard of care for hypertrophic scars, this method carries a risk of skin atrophy and hypopigmentation depending on the drug doses and concentration used. Administering corticosteroids via microneedle patch may improve delivery and, as a result, safety. Preliminary study results showed reduction in mean scar volume and improvement in Patient and Observer Scar Assessment Scale (POSAS) scores in the experimental portion of the scars.
In 2023, Chen et al. assessed the utility of a microneedle patch loaded with photosensitizers and chloroquine for visible light photodynamic therapy (PDT). A rabbit-ear scar model showed increased toxicity of fibroblasts and decreased collagen and transforming growth factor β (TGF- β) expression compared to application of traditional transdermal photosensitizer. In 2023, Wang et al. assessed interferon α-1b loaded on a self-dissolving polymer microneedle also in a rabbit-ear scar model. This treatment inhibited fibroblast and collagen deposition and decreased the color and thickness of the scar, with results comparable to those from intralesional triamcinolone injection. Although these preliminary results offer a promising method of drug delivery, further large-scale human studies are needed to better characterize safety and efficacy of microneedle patches for scar treatment.
Nanoparticles and 3D Printing of Skin
Nanotechnology-based scaffolds are structures designed with nanoparticles to mimic the extracellular matrix and play a key role in regenerative medicine. These materials offer a twofold benefit for scar management and wound healing: the scaffolds are constructed with biosimilar materials to optimize the wound-healing environment and can also be embedded with molecules to allow for improved scar formation. For instance, in 2018, Gholipourmalekabadi and colleagues studied use of an amniotic membrane embedded with adipose tissue–derived mesenchymal stem cells on a mouse scar model. This material resulted in improved scar healing and reepithelialization, along with earlier neovascularization. In 2022, Sharifi and colleagues tested a glass-ceramic-based scaffold loaded with mouse fibroblasts. In a mouse full-thickness wound model, this scaffold yielded nearly scarless wound healing with regeneration of sebaceous glands and hair follicles.
The latest innovation in wound care is 3D bioprinting of skin to allow for scarless wound healing. This technology produces biosimilar materials that can be loaded with human-derived stem cells similar to the nanotechnology-based scaffolds detailed above. Fu et al. evaluated use of 3D-bioprinted skin on a full-thickness mouse wound model. Compared to full thickness, partial thickness, and control groups, the 3D-bioprinted tissue yielded improved reepithelialization, angiogenesis, and scar appearance. In 2022, Fang and colleagues also designed a bioprinted lactic-co-glycolic acid-based membrane to target hypertrophic scars in a rabbit ear model. This material resulted in decreased collagen deposition and improvement in scar hypertrophy compared to controls.
Rapid innovation in the fields of gene therapy, nanotechnology, and regenerative medicine have allowed scientists to develop these novel treatments to improve scar outcomes. Further research and large-scale trials will allow us to expand our treatment repertoire for scars and wound healing.
Ongoing Clinical Trials
There are many ongoing trials for medical and interventional management of scars. Ongoing registered trials based in the United States are summarized in Table 12.1 .
| NCT Number | Study Title | Sponsor | Intervention | Primary Outcomes | Status |
|---|---|---|---|---|---|
| NCT05166395 | Laser Treated Scars and Optical Coherence Tomography (OCT) | University of Miami – Miami, FL | Er:YAG laser | Patient and Observer Scar Assessment Scale (POSAS), scar blood flow, scar skin roughness, scar collagen content, scar epidermal thickness | Not yet recruiting |
| NCT04456127 | CO 2 Laser Revision for Burn Related Donor Site Scars | Medstar Health Research Institute – Washington, DC | Fractional CO 2 laser | POSAS, Vancouver Scar Scale, (VSS), Delfin instruments (validated and calibrated skin measurement tools) | Recruiting |
| NCT04619589 | Characterization of Dyschromic Hypertrophic Scar | Medstar Health Research Institute – Washington, DC | None (observational) | Identify melanocyte presence via en face staining and melanocyte markers | Recruiting |
| NCT04364217 | Pain and Itch Reduction in Burn Scars Treated With Fractional CO 2 Laser | Massachusetts General Hospital – Boston, MA | Lumenis Ultrapulse fractional ablative carbon dioxide laser | Histologic change in nerve density | Recruiting |
| NCT03403621 | Hypertrophic Scar Prevention by Novel Topical Gel Application | Mayo Clinic – Rochester, MN | Topical Pentamidine isethionate, silicone gels | Serious and nonserious adverse events (including skin infection, irritation, and wound dehiscence) | Recruiting |
| NCT04176705 | Fractional Ablative Laser Treatment for Skin Grafts | Wake Forest University Health Sciences – Winston-Salem, NC | Fractional ablative laser | Skin graft contracture 90 days and 1 year post grafting | Recruiting |
| NCT04567537 | Ablative Fractional Laser Treatment for the Improvement of Hypertrophic Scars and Scleroderma: A Prospective Cohort Study | Massachusetts General Hospital – Boston, MA | Fractional ablative laser | Physician’s Global Assessment Scale for disease activity at baseline, and 1, 2, 3 months and 1 year posttreatment | Recruiting |
| NCT04807179 | Prospective Clinical Study to Evaluate the Efficacy and Safety of an Alexandrite Laser Device for the Treatment of Acne Scars | Cynosure, Inc | Noninvasive radiofrequency Alexandrite laser | Photographic Evaluation with échelle d’évaluation clinique des cicatrices d’acné (ECCA) grading | Recruiting |
| NCT03692273 | A Within-Scar, Randomized Control Trial Comparing Fractional Ablative Carbon Dioxide Laser to Non-Energy-Based, Mechanical Tissue Extraction and No Treatment | Massachusetts General Hospital – Boston, MA | Lumenis ultrapulse fractional ablative carbon dioxide laser | VSS | Recruiting |
| NCT04740268 | Clinical Trial Study for the Use of Straberi Microneedling Device to Treat Atrophic Acne Scarring | Universal Skincare Institute – New York, NY | Straberi microneedling device | Goodman and Barons quantitative scar scale, Global Aesthetic Improvement Scale (GAIS) | Recruiting |
| NCT05597267 | The MIRIA Acne Scar Study | AVAVA, Inc – Waltham, MA | AVAVA MIRIA laser skin system | ECCA, GAIS (physician and subject) | Recruiting |
| NCT05358860 | Safety and Efficacy of Sofwave Treatment for Acne Scars Appearance Improvement | Sofwave Medical, Ltd | Sofwave | Acne Severity Scale (ASS) | Recruiting |
| NCT05618912 | Scar Appearance After Postoperative Hydrocolloid Dressing Versus Standard Petrolatum Ointment: A Randomized Controlled Trial | Indiana University – Carmel, IN | Hydrocolloid dressing | Modified visual analogue scar scale (patient and physician assessment) | Recruiting |
| NCT05847530 | Pilot Evaluation of the Cynosure Potenza™ System for Treatment of Cosmetic Dermatologic Skin Conditions | Cynosure, Inc | Potenza (microneedle radiofrequency device); Icon Intense Pulsed Light (IPL) | Grading of skin aging and photodamage | Recruiting |
| NCT05897723 | Safety and Efficacy of Fractional Radiofrequency for the Reduction of Surgical Scar Formation | Venus Concept | Fractional Radiofrequency | GAIS, Manchester Scar Scale (MSS) | Not yet recruiting |
| NCT03294863 | Use of Embrace Device After Cutaneous Wound Closure: A Randomized Evaluator Blinded Split Wound Comparative Effectiveness Trial | University of California, Davis – Sacramento, CA | Embrace Scar Therapy Device | POSAS, scar cosmesis, width of scar | Recruiting |
| NCT05128383 | An Open-label Proof of Concept Study Regarding the Efficacy and Safety of Dupilumab in the Treatment of Keloids | Beth Israel Deaconess Medical Center – Boston, MA | Dupilumab | POSAS | Recruiting |
| NCT01619553 | Identification of Genetic Variants That Contribute to Keloid Formation in Families and Isolated Cases | UConn Health – Farmington, CT | None (observational) | Identification of genetic elements | Recruiting |
| NCT04853433 | Primary Radiotherapy for the Treatment of Keloids: A Pilot Study | Albert Einstein College of Medicine – Bronx, NY | Radiation therapy | Toxicity of radiation | Recruiting |
| NCT04722263 | Primary Radiotherapy for the Treatment of Keloids: A Pilot Study | Montefiore Medical Center – Bronx, NY | Radiotherapy | Incidence of adverse events | Recruiting |
| NCT02923596 | Retrospective Study of Keloid Disorder | Michael H. Tirgan, MD – New York, NY | None (observation) | Keloid recurrence rate | Unavailable |
| NCT03754218 | Phase 1 Study of Human Amnion Membrane Powder for Enhanced Wound Healing | Wake Forest University Health Sciences – Winston-Salem, NC | Amnion membrane powder | Incidence of donor site wound closure | Recruiting |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree