Fig. 8.1
Original figure of Günter Stüttgen’s publication (1964). adrenerg. adrenergic, cholinerg. cholinergic, Enzym. Akt. (Leber) enzyme activity in the liver, Ganglien ganglia, Gefässe vessels, Haut skin, Keimdrüsen sexual glands, Neuroleptica neuroleptic drugs, NNR adrenals, Parkinson-Syndrom Parkinson’s disease, Piloarrect. musculus arrector pili, Promethazin promethazine, Schweissdrüse sweat gland, Talgdrüse sebaceous gland, ZNS CNS
Among the several endocrine functions of the sebaceous gland currently reported (Zouboulis 2010; Table 8.1), its independent endocrine function and the involvement in a regulatory neuropeptide program are of major importance.
Table 8.1
Endocrine functions of the sebaceous gland
Synthetic activity |
Production of vernix caseosa |
Production of sebum |
Expression of the histamine-1 receptor and inhibition of squalene through antihistamines |
Endocrine properties |
Regulation of the independent endocrine function of the skin |
Expression of all enzymes that are responsible for steroidogenesis from the circulating lipids |
Regulation of local androgen synthesis |
Major role in the hormonally induced skin aging process |
Modification of lipid synthesis by a combined androgen and peroxisome proliferator-activated receptor ligands, estrogen, and the insulin-like growth factor-1 axis |
Expression of vitamin D receptor and the vitamin D metabolizing enzymes |
Expression of the retinoid metabolizing cytochrome P450 enzyme system |
Selective control of the action of hormones and xenobiotics on the skin |
Influenced by a regulatory neuropeptide program |
Hormone Receptors in Human Sebaceous Glands and Their Biological Activity
Sebocytes express receptors for peptide hormones, neurotransmitters, which are mostly arranged on the cell surface, and for steroid and thyroid class hormones (Table 8.2), which are found in the cytoplasm or nuclear compartment (Zouboulis 2009a; Fig. 8.2).
Table 8.2
Hormone receptors in human sebaceous gland cells
Receptors | Natural ligands | Function on sebocytes |
|---|---|---|
Peptide hormones and neurotransmitter receptors | ||
Serpentine receptors (seven transmembrane domain) | ||
CRH receptors 1 and 2 (CRHR1 > CRHR2) | CRH, urocortin | ↓ proliferation, ↑ Δ5–4 3β-HSD (CRH), ↑ lipogenesis (CRH), ↑ IL6 and IL8 release (CRH) |
Melanocortin-1 and -5 receptors (MC1R and MC5R) | α-MSH | ↓ IL1-induced IL8 synthesis (MC5R), differentiation marker (MC1R) |
µ-Opiate receptors | β-Endorphin | ↓ EGF-induced proliferation, ↑ lipogenesis |
VPAC receptors | VIP, neuropeptide Y and CGRP | Neuropeptide Y-stimulated IL6 and IL8 release |
Cannabinoid receptors (CR1 and CR2) | Cannabinoids | ↑ Lipogenesis |
Histamine receptor 1 | Histamine | Regulation of squalene synthesis |
Single transmembrane domain receptors with endogenous tyrosine kinase activity | ||
IGF-1 receptor | IGF-1, insulin | ↑ Lipogenesis |
EGF receptor | EGF | Controls differentiation (↓ adipophilin and MC5R levels), proliferation (↑), lipogenesis and inflammation (↓ IL6, IL8 and TNFα levels) |
Single transmembrane domain receptors without endogenous tyrosine kinase activity | ||
GH receptor | GH | ↑ Differentiation, ↑ 5α-DHT activity on lipogenesis |
Nuclear receptors | ||
Steroid receptors | ||
Androgen receptor | Testosterone, DHT | ↑ Proliferation (in association with PPAR ligands: ↑ lipogenesis) |
Progesterone receptor | Progesterone | |
Thyroid receptors | ||
Estrogen receptors (ERα and ERβ) | 17β-estradiol | ↑ Synthesis of polar lipids |
Retinoic acid receptors (RARα and RARγ) | all-trans-RA | ↓ Proliferation |
Retinoid X receptors (RXRα, > RXRβ, RXRγ) | 9-cis RA | Regulation of lipogenesis (?) |
Vitamin D receptor (VDR) | Vitamin D3 | Regulation of cell proliferation, cell cycle, lipid content, and IL6 and IL8 release |
Peroxisome proliferator-activated receptors (PPARα, PPARγ > PPARβ) | LA (RRARβ/d), LTB4 (RRARα) PG-D2, 15-deoxy-Δ12,14-PG-J2 (RRARγ) | ↑ Lipogenesis, ↑ PG-E2 release, ↑ IL6 release, ↑ COX-2 synthesis ↑ Lipogenesis, ↑ IL6 release ↑ Eotaxin 3 |
Liver X receptors (LXRα and LXRβ) | 22(R)-Hydroxycholesterol | ↓ Proliferation, ↑ lipogenesis, ↓ COX-2-induced nitric oxide synthetase |
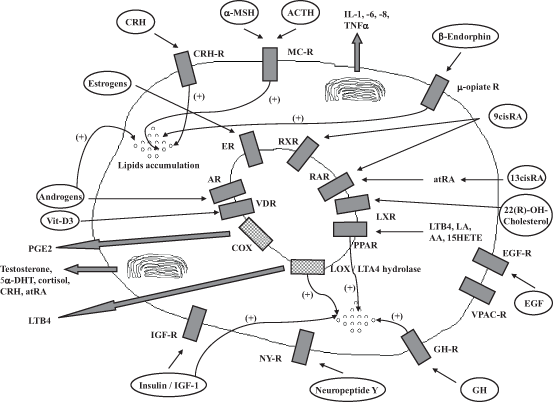
Fig. 8.2
Interaction between enzymes, membrane, and nuclear receptors as well as their ligands in human sebocytes: their influence on lipid accumulation is displayed. The release of various hormones and inflammatory mediators is also seen. α-MSH α-melanocyte stimulating hormone, 9-cis-RA 9-cis-retinoic acid, AA arachidonic acid, ACTH adrenocotricotropic hormone, AR androgen receptor, at-RA all-trans-retinoic acid, COX cyclooxygenase, CRH corticotropin-releasing hormone, DHT 5α-dihydrotestosterone, EGF epidermal growth factor, EGR-R epidermal growth factor receptor, ER estrogen receptor, GH growth hormone, IGF-1 insulin like growth factor-1, IL interleukin, LA linoleic acid, LOX lipoxygenase, LTA4-hydrolase leukotriene A4 hydrolase, LTB4 leukotriene B4, LXR liver X receptor, NY neuropeptide Y, PG prostaglandin, PPAR peroxisome proliferator-activated receptor, R receptor, RAR retinoic acid receptor, TNF-α tumor necrosis factor-α, VDR vitamin D receptor, Vit-D 3 vitamin D3, VPAC-R vasoactive intestinal peptide receptor. (modified from Zouboulis 2008)
Peptide Hormone and Neurotransmitter Receptors
Serpentine or “seven transmembrane domain” receptors, which are expressed and functional in human sebocytes, are:
CRH receptor (CRHR)1 and 2, whereas CRHR1 is more abundant and seems to regulate CRH activity (Zouboulis et al. 2002; Krause et al. 2007). Through binding to CRHR1, CRH and urocortin reduce sebocyte proliferation. CRH upregulates Δ5–4 3β-hyroxysteroid dehydrogenase expression, synthesis of neutral lipids and interleukin(IL)6 and IL8 release.
Melanocortin [α-melanocyte stimulating hormone (α-MSH)]-1 and -5 receptors (MC1R and MC5R), which bind α-MSH and are located at the cellular surface of sebocytes. MC1R regulates inflammation in SZ95 sebocytes (Böhm et al. 2002) and exhibits a stronger expression in acne-involved sebaceous glands (Ganceviciene et al. 2007). The expression of MC5R is weaker than that of MC1R but has been shown to be a marker of human sebocyte differentiation, since it is expressed in differentiated, lipid-containing sebocytes, only (Zhang et al. 2006a).
µ-opiate receptors, which bind β-endorphin. β-endorphin stimulates lipogenesis and specifically increases the amount of C16:0, C16:1, C18:0, C18:1, and C18:2 fatty acids to an extent similar to linoleic acid in sebocytes (Böhm et al. 2004).
VPAC receptors, which bind vasoactive intestinal polypeptide, receptors for neuropeptide Y, and calcitonin gene-related peptide (Seiffert et al. 2000). Neuropeptide Y activates cytokine synthesis. The calcitonin gene-related peptide is often colocalized with substance P.
Cannabinoid receptors (CBR) 1 and 2 are expressed in SZ95 sebocytes and sebaceous glands (Ständer et al. 2005; Dobrosi et al. 2008). CBR1 was found in the differentiated sebocytes and CBR2 in the undifferentiated cells, whereas endocannabinoids influence sebocyte differentiation via CBR2.
Histamine 1 receptor, which binds with histamine and regulates squalene synthesis (Pelle et al. 2008). Antihistamines, ligands of histamine 1 receptor reduced squalene synthesis in SZ95 sebocytes.
The single-transmembrane domain receptors, insulin-like growth factor (IGF)-1 receptor, and epidermal growth factor (EGF) receptor that harbour intrinsic tyrosine kinase activity are expressed on SZ95 sebocyte cell surface.
IGF-1 receptor can be activated by IGF-1 and high concentrations of insulin (Makrantonaki et al. 2006). It amplifies lipid accumulation in SZ95 sebocytes in a dose-dependent manner. The activation of the IGF-1 receptor induced lipogenesis in SEB-1 sebocytes by sterol response element-binding protein-dependent and independent pathways (Smith et al. 2006). IGF-1 also stimulates proliferation and differentiation of rat preputial gland cells, which resemble sebocytes, especially in combination with growth hormone (GH; Deplewski and Rosenfield 1999).
EGF receptor was shown to be expressed in human sebocytes (Nanney et al. 1984; Takata et al. 2012). Its inhibition in SZ95 sebocytes led to upregulation of adipophilin and MC5R expression levels, which are differentiation markers for human sebocytes, and enhanced proinflammatory signaling by induction of IL6, IL8, and tumor necrosis factor-α release. Current data indicate that EGF may play a multimodal role on sebaceous gland cell proliferation, differentiation, lipogenesis, and inflammatory signaling (Zouboulis 2013).
Activation of GH receptor, which does not possess intrinsic tyrosine kinase activity but appear to function through interaction with soluble transducer molecules, in SZ95 sebocytes, stimulates sebocyte differentiation and augments the effect of 5α-dihydrotestosterone (DHT) on sebum synthesis (Zouboulis et al. 2002; Makrantonaki et al. 2006).
Nuclear Receptors
The steroid receptor family is represented in human sebocytes by the androgen receptor (AR) and the progesterone receptor (PR), which are mostly expressed in basal and early differentiated sebocytes (Zouboulis et al. 2007; Fritsch et al. 2001; Fimmel et al. 2007).
Human sebocytes exhibit the highest AR density among human skin cells. AR down regulation reduces sebocyte proliferation (Fimmel et al. 2007). Five intracellular enzymes—all of them expressed in sebocytes (Fritsch et al. 2001)—are involved in activation and inactivation of androgens before binding to AR. Dehydroepiandrosterone (DHEA) sulfate is metabolized by the steroid sulfatase to DHEA. DHEA and androstosterone are converted to testosterone and later to DHT by 5α-reductase (Fritsch et al. 2001; Chen et al. 1998). Sebocyte studies of Akamatsu et al. and Zouboulis et al. showed a dose-dependent induction of sebocyte proliferation by testosterone treatment (Akamatsu et al. 1993) and no effect on lipid stimulation (Zouboulis et al. 1999). Investigations by Rosenfield et al. and Makrantonaki et al. proved that the effect of androgens on sebaceous lipids is mediated by peroxisome proliferator-activated receptor (PPAR) ligands (Rosenfield et al. 1998; Makrantonaki and Zouboulis 2007).
PR was found in nuclei of basal sebocytes of sebaceous glands (Pelletier and Ren 2004). The thyroid receptors, estrogen receptors (ER; α- and β-isotypes; Pelletier and Ren 2004; Thornton et al. 2006; Thornton et al. 2003), retinoic acid receptors (RAR; isotypes α and γ), and retinoid X receptors (RXR; isotypes α, β, γ; Reichrath et al. 1997; Tsukada et al. 2000), vitamin D receptor (VDR; Reichrath et al. 2000), PPAR (Makrantonaki and Zouboulis 2007; Schmuth et al. 2005; Alestas et al. 2006), and liver X receptors (LXR; -α and -β isotypes; Russell et al. 2007; Hong et al. 2008) are expressed in human sebocytes.
ER-β is expressed in basal and partially differentiated sebocytes. ER-α is expressed in basal and early differentiated sebocytes. One of the natural estrogens, estradiol, is created by oxidative reduction of 4-androstene-3,17-dione. Treatment of sebocytes with 17β-estradiol showed an effect on polar lipid production but no stimulating effect on neutral lipids (Makrantonaki et al. 2008). Other previous in vitro data indicated that estrogens may have an influence on the biological activity of sebaceous glands (Guy et al. 1996).
RARα and γ and RXRα are the predominant retinoid receptors in human sebocytes. RAR regulates cell proliferation (Tsukada et al. 2000). The natural ligands for RAR and RXR are all-tans-retinoic acid (RA) and 9-cis RA. 13-cis RA inhibits proliferation of SZ95 sebocytes, whereas it was found to be metabolized intracellularly to its isoform and RAR ligand all-tans-RA. RXR agonists (rexinoids) are stimulating sebocyte differentiation and proliferation. Rexinoids in combination with specific PPAR agonists, such WY 14643, troglitazone and cabaprostacycline, affected differentiation and growth in cultured primary sebocyte-like rat preputial cells (Kim et al. 2001).
SZ95 sebocytes were found to express vitamin D-25-hydroxylase, 25-hydroxyvitamin D-1α-hydroxylase, and 1,25-dihydroxyvitamin D-24-hydroxylase (24OHase; Krämer et al. 2009). Vitamin D3 induces time- and dose-dependent modulation of cell proliferation, cell cycle regulation, lipid content, and IL6 and IL8 secretion by cultured sebocytes. RNA expression of VDR and 24OHase was upregulated along with vitamin D3 treatment.
PPARα and γ are the predominant PPAR subtypes in human sebocytes (Alestas et al. 2006). PPAR is present in mitochondria, peroxisomes and microsomes of sebocytes, and regulate multiple lipid metabolic genes. Sebaceous lipogenesis is maximally induced by androgens and PPAR ligands (Makrantonaki and Zouboulis 2007).
SZ95 sebocytes express LXRα and β receptors at the mRNA and protein levels. The application of natural 22(R)-hydroxycholesterol or synthetic ligands significantly inhibited sebocyte proliferation and increased lipogenesis. The expression of known LXR targets, such as fatty acid synthase and SREBP1, was induced by the synthetic LXR ligand TO901317, which also decreased the expression of cyclooxygenase 2 and inducible nitric oxide synthase that was induced by lipopolysaccharide treatment (Hong et al. 2008).
Activation of the HPA-Like Skin Axis in Sebaceous Glands
Communication and reciprocal regulation between nervous, endocrine, and immune systems are essential for biological stability and responses to external and internal challenges. In particular, neuropeptides, hormones, and cytokines act as signaling molecules that mediate communication between the three interacting systems. Analogous to central responses to stress, which involve predominantly the hypothalamic–pituitary–adrenal (HPA) axis, it has been proposed that the skin may share similar mediators (Alesci and Bornstein 2000; Slominski and Wortsman 2000; Slominski et al. 2000), whereas the sebaceous gland plays a major role in these procedures (Zouboulis et al. 2002; Zouboulis and Chen 2013; Zouboulis and Böhm 2004; Zouboulis 2009b; Schagen et al. 2011; Elewa et al. 2012).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






