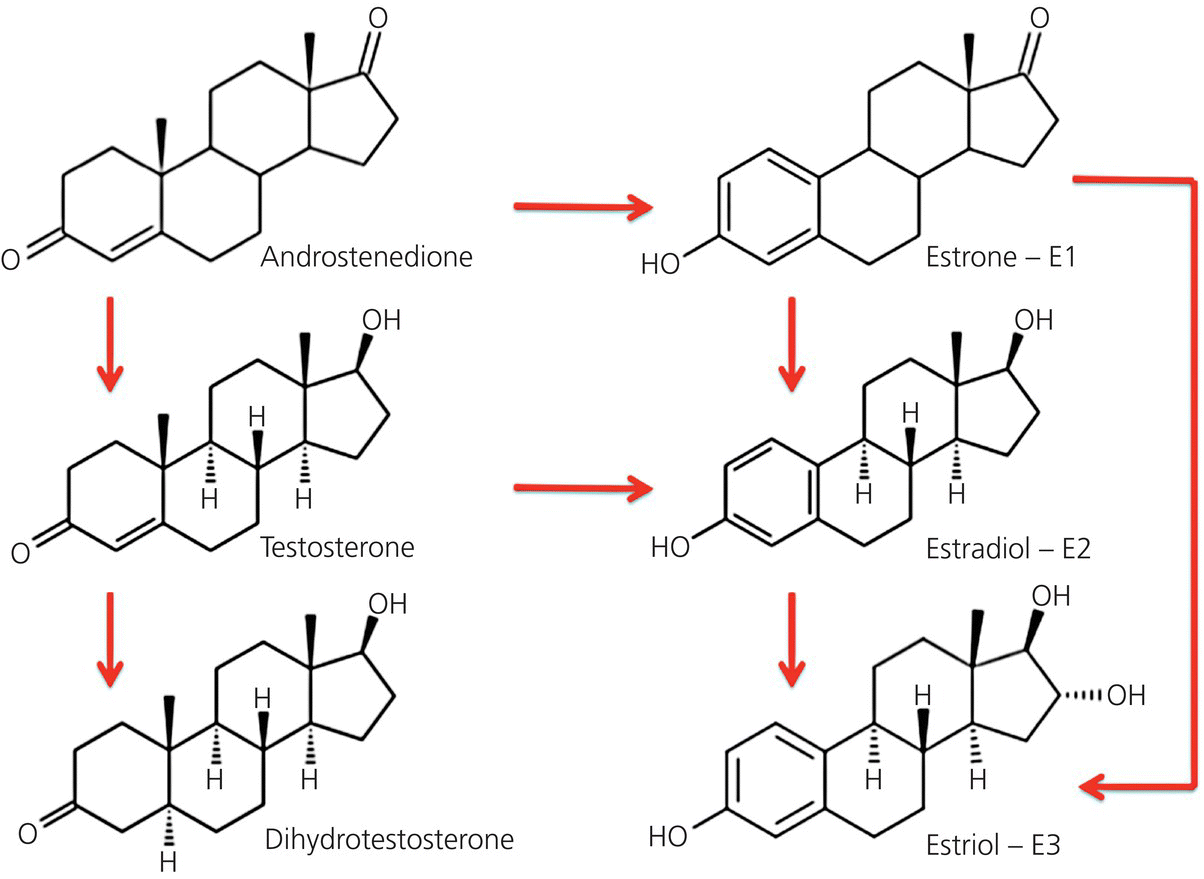
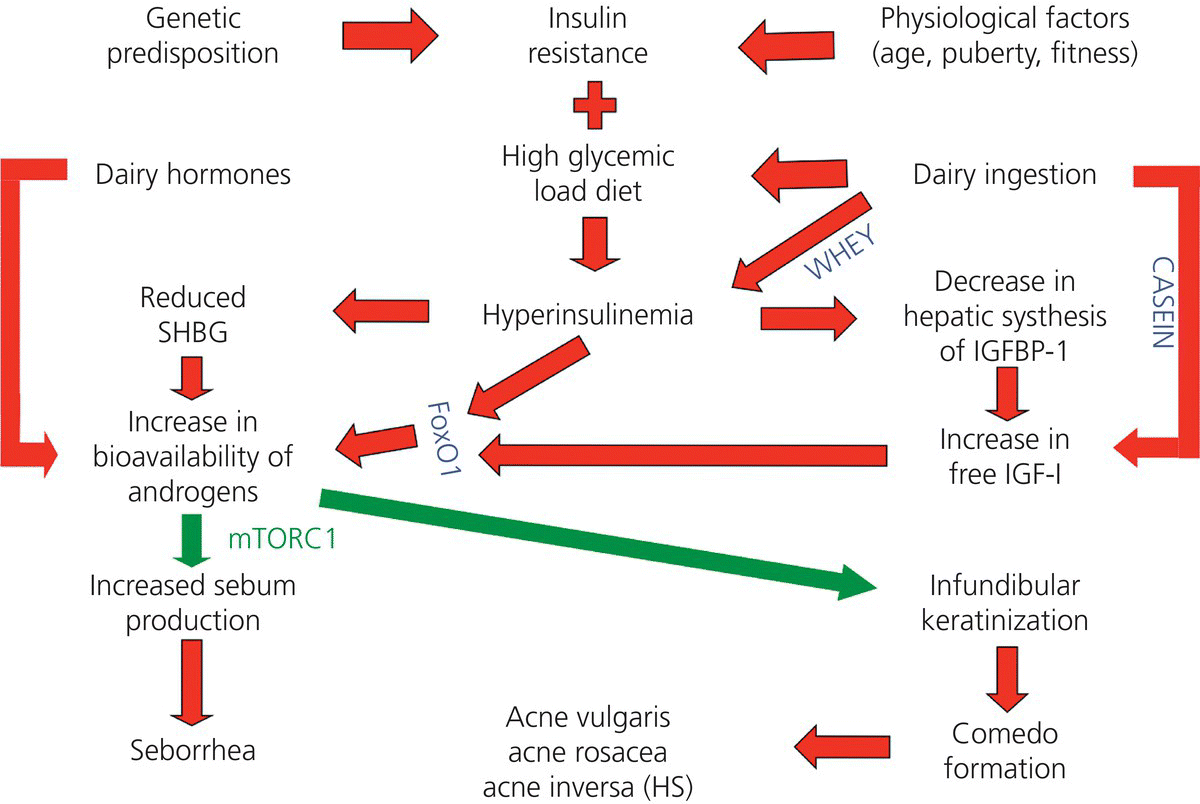
Chapter 4 The major stimulus to the development of acne is the male hormone dihydrotestosterone (DHT). It is a member of the family of steroid hormones, specifically one of the reproductive hormones, and in particular the most potent androgenic androgen. Many of these steroid hormones warrant the label of acnegenic because they generate acne. As explained in Sections 2.8 and 2.9, their actions are facilitated by other hormones and growth factors. These hormones are sourced both from within and from outside the human body. Endogenous means “generated within” and so this chapter will briefly review the hormones from sources within the body that impact acne, whether positively or negatively. The androgens are the male hormones. While they are male in effect, they are present in both males and females, with a much higher volume being produced in males. In women there are two sources, the ovaries and the adrenal glands. In males the specialized testosterone-producing Leydig cells in the testicles are the major source. They significantly outproduce the amount and potency of androgens produced by the adrenal glands. In both men and women, although testosterone (T) has a great deal to do with the development of the genital organs in utero, the major activity surrounding sexual maturity is driven by the 5α-reduced testosterone molecule, known as 5α-dihydrotestosterone, dihydrotestosterone, or DHT. The only difference between testosterone and DHT is the saturation of the double bond at position 5 of the steroid molecule. The double bond disappears, and a hydrogen atom is added at the 5α position, a small change that increases the power of the molecule by 10 times, and that has tremendous consequences. It is DHT that does all the “dirty work” that brings us dermatologists into the lives of our patients. Basically it is responsible for the acnes, for superfluous hair on the upper lip and elsewhere in women, and for balding in both sexes. In addition to the actual T molecule, there are a number of testosterone precursors (hormones that turn into testosterone) produced by the ovaries and adrenal glands. The overall picture is complex in women, with both testosterone and its precursor androstenedione produced in the ovaries, while dehydroepiandrosterone (DHEA) and its longer lasting sulfate (DHEAS) are produced in the adrenal glands. The percentage produced by each organ will vary somewhat, but the end result is a pool of testosterone and related androgenic molecules circulating in the blood. Some of this T is bound to sex hormone–binding globulin (SHBG), some of it travels free in the plasma (free T), and there is also a small amount of DHT circulating in the blood. There are some daily (diurnal) variations in both sexes. Monthly variations in women depend on the menstrual cycle if it is active. In general the levels are reasonably constant throughout the childbearing years in women. In men, the same is true, but with variations dependent on general health, exercise, diet, age, and of course the possibility of supplementation. Supplements, of course, are exogenous steroids. They may be used by either sex, and are dealt with in Section 4.2 . In women, the major source of estrogens is the ovaries. Estrone (E1), estradiol (E2), and estriol (E3) vary both in the amount produced by the ovaries and in their relative potencies. The subject is vast. For our purposes, we will stay with estradiol. It is the prime estrogenic actor in the acne story. The other source of estrogens, a minor one in women and a major one in men, is peripheral conversion (usually in fatty tissue) from testosterone. With the assistance of the aromatase enzyme, testosterone is transformed to estradiol, and androstenedione to estrone (Figure 4.1). Figure 4.1 Peripheral conversion of androgens to estrogens. The ovaries are the major source of the estrogens in females; these pathways to E1, E2, and E3 are more important in males. The major role of progesterone is the preparation for and promotion of gestation (pregnancy). Because of this, the major source, and it is a cyclic source, is the ovaries. When an ovary releases an egg (ovum), a yellowish collection of cells called the corpus luteum (the “yellow body”) is left behind at the site of release of the ovum. These cells are the source of the progesterone and other hormones that prepare the lining of the uterus (the endometrium) for pregnancy. They also stimulate the breasts on a monthly basis to prepare for pregnancy. These are solid monthly reminders that breast tissue and the folliculopilosebaceous unit (FPSU) share a common origin as epidermal appendages and are controlled by the same family of hormones. The increase of facial oiliness at “period time” is familiar to most women. This active oil gland output is caused by the same hormones that produce the breast swelling and sometimes tenderness. When no pregnancy occurs, the corpus luteum normally simply melts away, and the progesterone and other ovarian hormone levels drop to baseline levels, waiting for another cycle to start. If a pregnancy does occur, the corpus luteum continues producing progesterone and other hormones at levels sufficient to sustain the pregnancy until the placenta can take over that role. Occasionally, even when no pregnancy occurs, the corpus luteum fails to melt away. This creates a persistent corpus luteum or even a corpus luteum cyst, leading to persistent production of these acnegenic hormones. This situation is occasionally identified at ultrasound examination as the cause of an isolated and otherwise unexplained exacerbation of acne. It usually resolves spontaneously. Progesterone is not the only hormone produced by the ovary in a cyclic fashion. In addition to androstenedione, dehydroepiandrosterone, and testosterone, a little-known product is 5α-pregnanedione (5α-P) [1]. As an aside, it is important to note that 5α-P is also responsible, in tissue culture, for inducing breast cancer cells to produce increased numbers of estrogen receptors on their cell surfaces, up to two and a half times the normal number, perhaps setting the stage for breast cancer to proceed [2]. In addition, 5α-P is one of the 5α-reduced hormones present in cow milk, another reminder of the common origin of human breasts, cow udders, and the FPSUs. It also provides a basis for speculation that perhaps the FPSUs are stimulated by the same mechanism, 5α-P, priming the human sebocytes to produce estrogen receptors, increasing the sebocytes’ response to ingested exogenous dairy estrogens, a subject worthy of laboratory investigation. While progesterone is a weak acnegen on its own, it also serves as a precursor for a complex cascade of other acnegens. (See Figure 2.12.) It is tempting to consider reducing the progesterone load to minimize acne, but the collateral damage to the androgen cascade and the reproductive system would be unacceptable. Until recently, the only relationship of insulin to the acnes was the known increase in general risk of infection and decreased speed of healing in the acne of diabetics. Insulin’s central role in acne has now been recognized and defined [3]. This is not a matter of acne being linked to diabetes, but is instead reflective of the fact that insulin is an important actor in many of our everyday metabolic processes, including those that lead to acne. Humans evolved to exist on what they found to eat in the world around them. Essentially that included fish and seafood, meat, fowl, eggs, berries, nuts, edible leaves, tubers, fruit, and vegetables. Originally, food was eaten raw. Insulin and insulin-like growth factor 1, both induced by maternal breast milk, were essential stimuli of neonatal growth and development until weaning. Thereafter, insulin served to finely tune plasma glucose levels, the function for which it is best known now. Eventually, cooking changed previously indigestible complex carbohydrates to a form the human body could more easily digest. The need to handle these simpler carbohydrates made the role of insulin much more important. Insulin’s job changed in the face of increasing amounts of dietary glucose. It became the facilitator of the entry of glucose into various cells, for both energy and storage, thus lowering the level of glucose in the blood. As we learn more of insulin’s quiet role in the background before we learned to cook, herd milk cows, and grind grain to fine flour, it is interesting to speculate that insulin has never really evolved as an adequate control mechanism to cope with the vast changes in our diet in the past 15,000 years. The capacity of each food to produce a rise in blood glucose is measured as the glycemic response. Eating pure sugar produces a very quick rise in blood glucose so the glycemic index (GI) of glucose is high (100), just as the GI of water is low (0). The more easily a carbohydrate-based food is digested and converted to sugar, the higher will be its GI. One can measure the glycemic response to a fixed weight (usually 100 g) of a solitary food or of a prepared dish by measuring the rise in blood sugar in the hours after the food is consumed, and thus calculate the GI. This then allows one to multiply the GI by the mass/weight of the various components of the food (or of the complete meal) to generate the glycemic load (GL). Small amounts of low-GI foods will have a minimal calculated GL, and large amounts of high-GI foods will yield a very high GL, with an obvious range of response between these two extremes. This rise in sugar in the blood triggers a rise in serum insulin that is infinitely variable. Because one of the main triggers of androgen receptor receptivity is insulin, this variation translates into a direct but variable relationship between the GI of individual foods, the GL of meals, and the subsequent androgenic response to foods. Insulin modulates the androgen receptor by inducing the phosphorylation of an intranuclear polypeptide called FoxO1, as described by Melnik [4]. Although it is essentially theoretical, the logical progression of his explanation combined with the experimental documentation of each of the independent metabolic steps are quite compelling. Each link in the argument strengthens the overall explanation he offers, and no logical arguments have been forthcoming to counter his explanations or to suggest alternatives. Briefly and in summary, insulin and IGF-1 (see Section 4.2.4.1 ) together synergistically induce a process that de-represses (opens up) the androgen receptor. By so doing, this combination of polypeptide hormones exposes the normally hidden (repressed) androgen receptor, forcing it to become receptive to androgenic molecules. This not only grants access to the endogenous androgens (and their precursors) in both sexes but also welcomes the exogenous androgens and androgenic precursors present in dairy products, plus other exogenous steroid sources such as birth control hormones and even performance-enhancing steroid supplements. Insulin thus appears to be one of the twin keys to the door that leads to the acnes. Aside from injectable insulin used in the management of diabetes, there is no known exogenous source, the sole exception being very small amounts in dairy-based foods and beverages. The inability to bring an orally administered insulin to market is testimony to the likelihood that the effect (in adults) of orally absorbed insulin is minimal or nil, due to digestion of the insulin molecule. So, how does all this fit together? Over the past 20 years or so, it has become apparent that, in the group of female patients who have difficulty with acne associated with irregular periods, infertility, ovarian cysts, hirsutism (excess hair), and weight control challenges, there is an additional problem called insulin resistance. This is part of a complex group of metabolic changes that has been given a number of different names, including the cryptic syndrome X. The cause of this disorder, usually now referred to as metabolic syndrome, is not totally sorted out, but it is important to consider that one of the abnormalities that appears regularly in the fully developed metabolic syndrome, the elevated level of insulin, predisposes to chronic androgen receptor de-repression. Thus, the chronically elevated insulin leads in turn to chronically “available” androgen receptors, leading to the cutaneous and ovarian signs of the disease. The phenomenon termed insulin resistance, through a mechanism that is not yet understood, elevates the circulating levels of insulin to a degree that can be easily measured, allowing fairly easy diagnosis. Therapy is not so easy. If one does not correct the intake of excessive calories, the insulin level slowly climbs higher. This appears to be part of the link of high-glycemic-load food to the induction and promotion of acne in this special situation. It may also help to explain the results in studies linking acne to high-glycemic-load diet and to elevated body mass index (BMI) [5]. These prolonged elevated insulin levels have the effect of opening the androgen receptors. This means that androgens that previously had been floating in the blood, looking for a place to go, are able to easily access the newly opened androgen receptors, “hook up” with them, and stimulate a host of responses. Although the most obvious impact of the androgen surge affects the classic hormone-responsive tissues, it is essential to remember that these steroid hormones, besides being involved in reproduction, are also broadly anabolic. This activation of the androgen receptor, through the subsequent activation and direction of mammalian target of rapamycin complex 1 (mTORC1) (see Section 2.9), stimulates growth of many nonreproductive tissues. That includes muscle and bone, which is why anabolic steroids are used as performance enhancers by many athletes, including race horses. In turning on the growth of these tissues, androgens contribute to the overall set of body changes that have been referred to in past years as Stein–Leventhal syndrome. This is the old terminology for what we now recognize as the collection of prominent markers of polycystic ovarian syndrome (PCOS). Although the impact on the acnes is the main subject here, overstimulation of scalp hair growth is also part of the picture and results in female pattern hair loss, for the same reason that men blessed (or cursed) with high testosterone levels tend to early baldness. The same excess of androgen also causes the “superfluous” hair on the face, particularly on the upper lip, and on the trunk, the breasts, the lower abdomen, and the inner thighs in women, all of which are involved to a variable extent in PCOS patients. Although acne is a marker for this disease, there are numerous other tissues impacted as well, particularly the ovaries, all stimulated by an aberrant and interactive testosterone and insulin metabolism. In Type I (classic early-onset) diabetes, there is a quickly decreasing level of insulin in the early stages of the disease and glucose metabolism is paralyzed by the lack of insulin. In the metabolic syndrome, the problem lies elsewhere. The source of insulin in this situation, basically an early manifestation of Type II diabetes, is the pancreas. But in Type II there is too much insulin, and this is most likely a product of dietary insults (outlined in this chapter) that essentially overwhelm insulin’s ability to cope, an inability that, as speculated here, may be due to there having been inadequate time for insulin control mechanisms to evolve to handle our calorie-laden modern Western diet. It appears that whey proteins ingested in milk are a part of the problem. These proteins are the most potent inducers of a polypeptide called glucose-dependent insulinotropic polypeptide (GIP) that is secreted in the gastrointestinal tract by neuroendocrine K cells. GIP works together with dietary-sourced essential amino acids derived from intragastric hydrolysis (digestion) of whey in milk products. This combination of amino acids and GIP stimulates chronic insulin secretion by pancreatic beta cells, and that is how milk and milk products stimulate the rise in the level of insulin [3]. High-glycemic foods, on the other hand, increase the levels of sugar. While this should be expected to induce more insulin to return the blood levels to normal, chronic hyperglycemia can itself impair pancreatic beta cell function and exacerbate insulin resistance, leading to a vicious cycle of hyperglycemia and causing a worsening metabolic state [6]. The major unanswered question here is the mechanism of insulin resistance. Searching the literature for a solid answer is an exercise in frustration. There are few subjects surrounded by such a high and fuzzy wall of inexplicability. Several possibilities are being explored, including: While it is tempting to speculate that the most highly consumed “unnatural” foods and their derivatives might be at fault, is there any reasonable alternative to the likely culpability of milk and dairy products, with or without a high-glycemic-load diet? What alternatives exist for consideration? Pesticides, fungicides, antibiotics, and other industrial and agricultural chemicals are possibilities, but the molecular mechanisms that link our modern diet to this group of conditions is becoming more readily understood and better explained year by year. In simple terms, the new chemicals are poisons and are likely to inhibit biologic processes. Our modern food, on the other hand, is producing disease by overstimulating normal metabolic processes. In a thorough discussion of the effect of diet on the most common disorder elevating the general population’s insulin levels, the metabolic syndrome, Unger and Scherer state, Based on evidence reviewed here, it seems that prevalent forms of metabolic syndrome and type 2 diabetes mellitus (T2DM) result from unremitting caloric surplus complicated by failure of adipocytes to maintain protection against lipotoxicity. If one imagines the US population to be unwitting volunteers in the largest (300 million subjects) and longest (50 years) clinical research project in history, the specific aim of which was to determine if the deleterious effects of sustained caloric surplus in rodents also can occur in humans, the outcome of the project becomes clear—after 50 years of exposure to an inexpensive calorie-dense diet high in fat and carbohydrates, 200 million subjects are overweight and over 50 million have metabolic syndrome. The failure of healthcare providers and pharmaceutical industries to contain the pandemic suggests that elimination of “bargain basement” calories will be required to “price obesity out of the market.” Unfortunately, this would have profound socioeconomic implications: how do we tax excessive calories while at the same time guaranteeing sufficient access to high quality foods for the underprivileged? [7] Whatever the cause, weight loss and a low-glycemic-load diet plus a zero-dairy diet minimize the risk, and can reverse the process. Studies of severe caloric restriction (600 calories per day for weeks) have recently shown promise in returning diabetics’ serum insulin levels to normal [8], but such a restrictive diet will be a tough sell to the general public. The other molecule that leads to de-repression of the androgen receptor is insulin-like growth factor 1 (IGF-1). It is produced by the liver as a result of stimulation by growth hormone (GH) from the pituitary. Together with insulin, as detailed in Section 2.9, IGF-1 triggers the de-repression of the androgen receptor, setting the stage for the development of acne. Just as was shown with insulin, the blood level of IGF-1 can be raised by ingestion of milk, and the casein component has been demonstrated to be responsible [9]. Other than therapeutic injection of GH for dwarfism, there is no known exogenous source. There is a very close relationship between the incidence of acne and the level of IGF-1 during puberty and adolescence. This far more closely approximates a reasonable causal relationship of acne to IGF-1 than has ever been demonstrated to any androgen (Figure 4.2) [10]. What’s more, recent work demonstrates that IGF-1 deficiency prevents the occurrence of acne [11]. Figure 4.2 The green line at the 250 level gives a remarkably accurate “acne threshold” for both the prevalence and the timing of acne vulgaris. In summary, insulin and IGF-1 are the “dynamic duo” in the chain of events that lead to the acnes. Controlling the endogenous stimuli that lead to their elevation is one-half of the battle for control of the forces that cause the acnes. Any hormone that originates outside the body, whether or not it is identical to an endogenous hormone, must be considered exogenous. The list is long, and growing. These are the steroids that are taken by or given to bodybuilders and athletes in order to produce superior performance (Figure 4.3). The word anabolic comes from the Greek word anabole, meaning “that which is thrown up; a mound.” Certainly, mounds of muscles (and medals) can be created, using what is essentially too much of a good thing. The general public and news readers are generally unaware that anabolic steroids are the normal everyday growth stimulants in our mothers’ milk, the food that starts each of us on the road from infancy to maturity. Figure 4.3 This list comprises the exogenous anabolic androgenic steroids, the man-made or man-modified hormones used to enhance performance (or to treat some diseases). Some of the endogenous anabolic androgenic steroids (androstenediol, androstenedione, dihydrotestosterone, dehydroepiandrosterone [DHEA], and testosterone) may also be used by injection and are then considered exogenous. Let’s start with a little-known fact. Anabolic steroids were part of your first meal. Our mothers’ milk contains anabolic steroids. They are an essential part of the stimulus to growth that turns a baby into a self-sufficient individual. They are totally natural, easily absorbed, impressively effective, uniquely tuned to the species of the mother, and, depending on your mother’s diet, probably “organic.” Nobody injected your Mum with steroids or growth hormone to make her produce more milk. These anabolic steroid hormones are provided in nature as part of what is intended as a temporary source of complete nutrition. Milk is indeed nature’s “perfect food,” but it wasn’t intended to be continued after the offspring had learned to adapt to a full childhood and adult diet. As Melnik has written, “We have to appreciate that milk is a species-specific endocrine signalling system that activates a central signalling node in cellular metabolism for stimulation of growth and cell proliferation.” Furthermore, The endocrinological changes in milk signalling are … comparable to the endocrinology of puberty. Both periods of growth, the milk-driven period of neonatal growth and growth hormone-driven puberty are associated with elevations in IGF-1, insulin and insulin resistance. [12] In all mammalian species, the nutrient-sensitive kinase mTORC1 integrates nutrient signals, such as glucose (adenosine triphosphate/energy status of the cell), essential amino acids (predominantly leucine availability), and growth factor signals (insulin, IGF-1, and fibroblast growth factors [FGFs]). Puberty-induced growth and milk-induced neonatal growth are driven by the same insulin and IGF-1 signal transduction pathways, which ultimately upregulate mTORC1 signaling to make things grow. FPSU overgrowth is highly likely to occur as the result of being overstimulated through this pathway, but although the “high glycaemic load pathway to mTORC1 in acne appears to be established, … the nutrient signalling of high milk/dairy protein consumption awaits further experimental confirmation” (Figure 4.4) [13]. Figure 4.4 The final effector, mammalian target of rapamycin complex 1 (mTORC), gives the green light to growth, causing the plugged pores that are at the root of all acnes plus the seborrhea that accompanies some varieties. We have all seen nature films in which the mother deer or cow is chasing away her growing offspring, discouraging further nursing as part of the weaning process. Weaning is natural; it is nature’s plan. Humans are the only species who consume the milk of other species, or who encourage house pets to do the same. I occasionally remind teenaged patients that, if Mother Nature intended them to be still drinking milk, they would have a closer relationship with their maternal parent. There are a number of substances in mothers’ milk that stimulate growth, but only some of them are exogenous steroidal hormones. These are dealt with in Section 4.2.4 . Milk contains numerous nonsteroidal hormones and growth factors [14] plus the fats, proteins, and carbohydrates that provide the energy and material for growth. But once a human baby or other young mammal is capable of consuming and digesting a standard diet, weaning in nature (and in civilization) should occur, despite the ability of some of us to consume milk over our entire lifetimes, and despite the temptations of ice cream sundaes and artisanal cheeses. The accident of nature that led to the genetic mutation that codes for lactase persistence arose several millennia in the past, apparently in what is now Central Europe. This permits individuals who would be otherwise lactose intolerant to continue to consume lactose-containing milk. It is argued that this mutation allowed early humans to take advantage of domesticated cattle and so survive in early Europe. While this may be considered a successful adaptive gene, it can be argued that this is really not a survival gene. If it were, drinking milk to ensure the propagation of the species would improve fertility or provide some reproductive advantage. As for drinking milk universally enhancing fertility, this is clearly not the case—millions of lactose-intolerant and milk-allergic individuals avoid milk and other dairy products and have babies every year. In fact, infertility was 85% higher in women who consumed two or more servings of low-fat dairy milk compared with those who consumed one or less servings per day. High-fat daily milk consumption gave opposite results [15]. Not all adaptations that human evolution has brought us are to our benefit, nor are we physicians free of blame, exposing our patients to man-manipulated hormones as we trade the loss of a natural, organic, and hormone-free existence for the expediency of effective birth control. Life is a trade, with costs, savings, risks, and benefits vying for consideration. The provision of anabolic steroids to athletes is a huge business. There is a vast array of steroids available. They are used singly and “stacked” in various combinations both orally and by intramuscular injection. A few minutes spent on the Internet will enlighten you more than I intend to do here. I am personally surprised that we see so few patients with acne triggered by nonprescription use of these potent molecules. I suspect this is because the users are aware that acne is a known side effect, and those who suffer from acne either stop the use of steroids on their own or prefer to avoid contact with dermatologists and other physicians who are known to be critical of such practices. On the other hand, the few patients I have seen with this problem actually improved while spending time on my waiting list, having already made their decision and having already discontinued the drugs. In general, the anabolic steroid users tend to be in their 20s and 30s, so they may be protected to some extent by their lower post-adolescent endogenous levels of IGF-1. But sometimes the chemically induced acne gets seriously out of hand, producing truly horrendous and destructive variants of acne [16]. The dermatologist’s role has generally been to clean up the residual. Only once (so far) has a resistant personal case of acne proven to be due to continued use of steroids. The confession occurred only after several months of frustration on my part. The message to physicians is clear—keep asking, and forbid whey and casein! Our world has been seriously rocked by the invention and introduction of this class of medications, particularly those used as oral contraceptives. We are now experiencing the fourth generation of “the Pill,” and there may be more to come. The original birth control pill (BCP) was intended to be a progestagen (progestin)-only preparation. Estrogens were not originally intended as part of BCPs. Curiously, an estrogenic substance, mestranol, was found as a contaminant during the development of the strongly progestagen/progestin-dominant preparation that was eventually to be marketed as Enovid®. The mestranol was found to prevent breakthrough bleeding and so, once an optimal dose had been established, the original preparation as sold contained 150 μg mestranol (the estrogen) and 9850 μg norethynodrel (the progestin/progestagen) for a total hormone dose of 10,000 μg or 10 mg, a much higher dose than is prescribed today. During the five decades since, the estrogen used has been generally standardized and is usually ethinyl estradiol (EE2). The dose has gradually been reduced as low as 10 μg per tablet. While this lower dose is usually sufficient estrogen to have the desired effect in preventing breakthrough bleeding, it does not provide the major contribution to acne control of the former higher doses. Where acne control is a consideration, higher doses of estrogen are needed, especially in young women weighing 55 kg (110 lbs.) or more. The dose of the EE2 needs to be raised for this population or they will risk an unacceptable incidence of breakthrough bleeding (as was the case with the original BCP before the mestranol was added). There is limited flexibility available in choosing doses for “the pill,” but the subject deserves consideration by the prescriber. Just as women vary by weight, they vary by age. Acne is not a disease restricted to women younger than 35 years of age. In the past decade, one of the many results of the scare induced by the Women’s Health Initiative in the United States (and similar initiatives elsewhere) has been the re-emergence of what is best termed acne climacterica, or menopausal acne. As women’s natural endogenous estrogen production fades around the late 40s and early 50s, testosterone’s effect becomes more and more dominant, being relatively unopposed by less and less estrogen. This androgen-dominant environment causes a variant of late-onset acne. Sometimes insult is added to injury, and hirsutism, androgenic alopecia, and numerous nondermatological complaints are added to the mix. Exogenous bio-identical estradiol administered as a patch is the safest, and most easily regulated, way of handling this estrogen deficiency. Bio-identical micronized progesterone, available as oral Prometrium® or as a generic, is used at the same time to support the health of the uterus, if it is still present. These bio-identical estradiol patch and oral progesterone preparations are preferred because they avoid the use of nonhuman and manufactured equivalents. Careful counseling and coordination with primary care physicians, gynecologists, and endocrinologists are advised. Fortunately, papers showing the advantages of hormone replacement therapy (HRT), when commenced early and when composed of less troublesome molecules, are beginning to appear [17]. Updated guidelines for HRT and reconsideration of the data that led to tens of millions of women (and their doctors) being frightened away from HRT are now available [18, 19] and should in time make this growing and multifaceted problem less common. Meanwhile, see the “Warnings” at Section 8.6.1.1. The original progestin used in BCPs was norethynodrel. Over the years, a trend to less androgenic progestins has made BCPs less acnegenic but there are still many androgenic progestins in use, including levonorgestrel, dl-norgestrel, and desogestrel. In choosing oral contraceptives with a view toward managing acne, the least androgenic progestin possible is needed, and is best combined with a reasonable dose of estrogen. The 20 μg dose, the “low dose” of the estrogen ethinyl estradiol, in the product containing 3 mg drospirenone, is normally sufficient for women weighing less than 50 kg (110 lbs.). Over that weight, we prefer to see the 30 μg dose used. The same general approach is used in other products containing different progestins (Table 4.1 and Figure 4.5). Table 4.1 Progestins vary, the more androgenic make acne worse, the less or non-androgenic prevent and clear acne.
The acne hormones
4.1 The endogenous hormones
4.1.1 Androgens and their sources
4.1.2 Estrogens and their sources
4.1.3 Progesterone and the progesteroids
4.1.4 Insulin
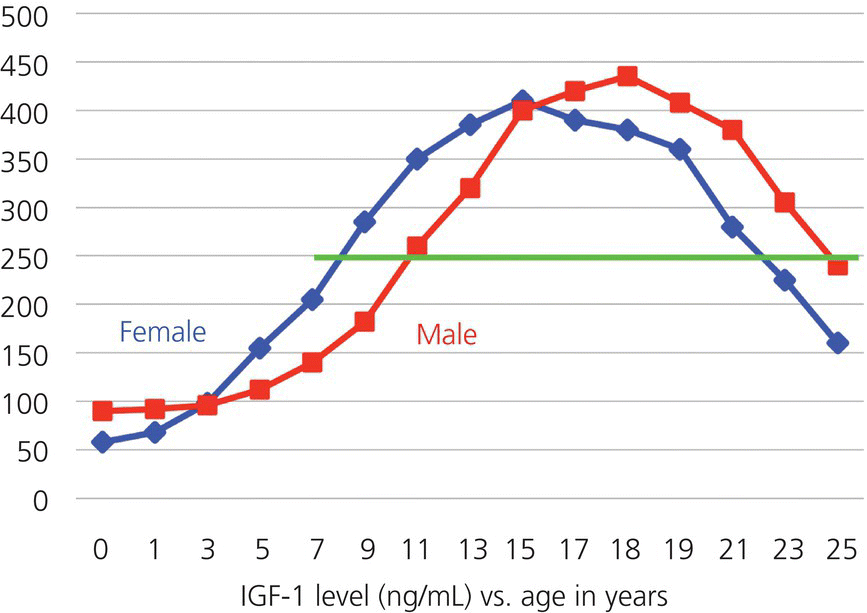
4.1.5 Growth hormone and insulin-like growth factor-1
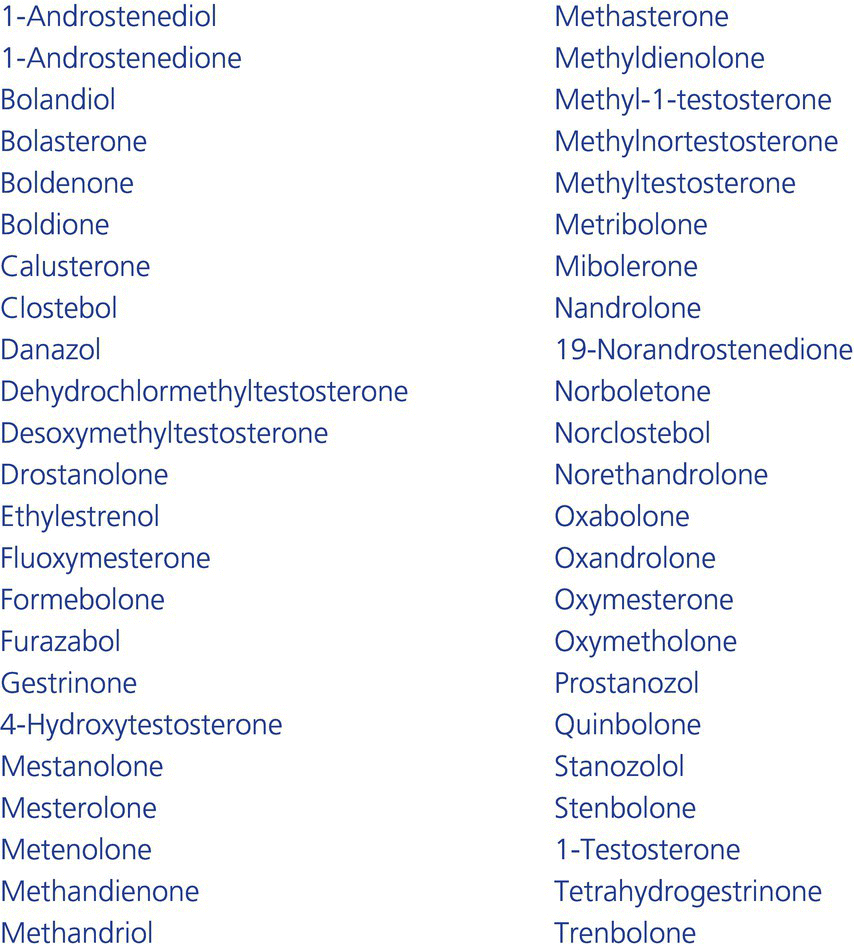
4.2 The exogenous hormones
4.2.1 Anabolic steroids
4.2.1.1 Mothers’ milk
4.2.1.2 Muscle makers
4.2.2 Oral contraceptive hormones
4.2.2.1 Oral estrogens
4.2.2.2 Oral progestins
The acne hormones
Source: Dickey RP. Individualizing oral contraceptive therapy.
< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree






