The transaxillary approach to breast augmentation has the advantage of allowing breast implants to be placed with no incisions on the breasts. There has been a general perception of a lack of technical control compared with the inframammary approach. This article presents the transaxillary approach from the perspective of the technical control gained with the aid of an endoscope, which allows precise creation of the tissue pocket with optimal visualization. The aspects of technique that allow optimal technical control are discussed, in addition to postoperative processes that aid in stabilizing the device position and allow consistent and predictable outcomes.
Key points
- •
The transaxillary approach to breast augmentation has the advantage of allowing breast implants to be placed with no incisions on the breasts.
- •
The approach has been criticized because of a general perception of a lack of technical control compared with the more widely favored inframammary approach.
- •
This article presents the transaxillary approach from the perspective of the technical control gained with the aid of an endoscope, which allows precise creation of the tissue pocket with optimal visualization.
- •
The components and aspects of technique that allow optimal technical control are covered in detail, in addition to postoperative processes that aid in stabilizing the device position and allow consistent and predictable outcomes.
The transaxillary approach to breast augmentation has the appeal of allowing breast implants to be placed with no incisions on the breast. However, the technique has met with long-standing resistance among plastic surgeons because of a perceived lack of technical control compared with the more universally accepted inframammary approach. This resistance is likely the result of the earliest reports of the technique that relied on blunt dissection performed in a largely blind fashion. There have been recent reports, however, that suggest this to be a reliable technique in large, single-surgeon experiences with long-term follow-up. In addition, higher patient satisfaction scores have been associated with use of the transaxillary incisions compared with visible-incision approaches, suggesting that this approach may warrant a more careful evaluation.
The addition of endoscopic assistance, as reported by Price and colleagues in 1994, allowed for direct tissue visualization and provided an improved level of technical control previously unseen with the transaxillary approach. This series detailed the use of endoscopic assistance in the placement of saline implants in a partial subpectoral pocket, at a time when silicone gel breast implants were under restriction by the Food and Drug Administration in the United States. Strock has reported on the technique of transaxillary placement of smooth silicone gel devices in a partial subpectoral pocket with endoscopic assistance. Additional reports from multiple investigators around the world have shown consistent and predictable outcomes with multiple device types through a transaxillary endoscopic approach. The author outlines his current approach to transaxillary breast augmentation, with emphasis on technical refinements that provide consistent technical control with this approach.
Preoperative planning
The incisions are marked preoperatively with the patient in a sitting position. The incision design and length differ depending on the type of device to be used. For saline devices, a 2.5-cm incision is made in an existing skin crease in the apex of the axilla. For silicone gel devices, a 5-cm incision is made, also centered in the axillary apex. The markings are started with a small mark made in the center of the axillary apex, in an existing skin crease. This mark is extended anteriorly toward the posterior aspect of the pectoralis major muscle, stopping just short of the posterior muscle border. From the initial mark in the center of the axillary apex, the incision is extended in a posterior direction, beveled superiorly, which allows the incision to not be visible when the patient stands with her hands on her waist ( Fig. 1 A). Alternatively, a straight incision can occasionally be used when a small volume device is to be placed in a patient with a long skin fold in the axilla.
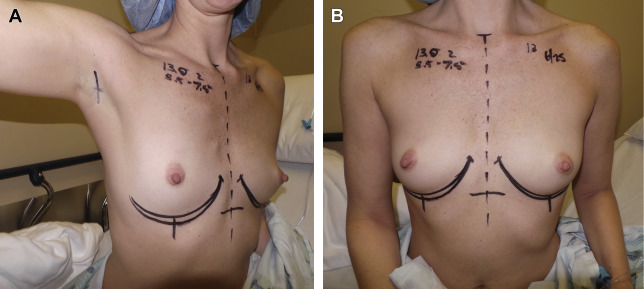
Additional markings focus on the inframammary fold, to define its preexisting level and shape, compared with the intended level and shape created during tissue pocket dissection and device placement. These markings are made with the patient in the sitting position preoperatively, and confirmed with the patient in the supine position at the start of the procedure. This method allows for a precise ability to confirm technical accuracy and symmetry intraoperatively following device placement (see Fig. 1 B).
Preoperative planning
The incisions are marked preoperatively with the patient in a sitting position. The incision design and length differ depending on the type of device to be used. For saline devices, a 2.5-cm incision is made in an existing skin crease in the apex of the axilla. For silicone gel devices, a 5-cm incision is made, also centered in the axillary apex. The markings are started with a small mark made in the center of the axillary apex, in an existing skin crease. This mark is extended anteriorly toward the posterior aspect of the pectoralis major muscle, stopping just short of the posterior muscle border. From the initial mark in the center of the axillary apex, the incision is extended in a posterior direction, beveled superiorly, which allows the incision to not be visible when the patient stands with her hands on her waist ( Fig. 1 A). Alternatively, a straight incision can occasionally be used when a small volume device is to be placed in a patient with a long skin fold in the axilla.
Additional markings focus on the inframammary fold, to define its preexisting level and shape, compared with the intended level and shape created during tissue pocket dissection and device placement. These markings are made with the patient in the sitting position preoperatively, and confirmed with the patient in the supine position at the start of the procedure. This method allows for a precise ability to confirm technical accuracy and symmetry intraoperatively following device placement (see Fig. 1 B).
Patient positioning and setup
Following the induction of general anesthesia, the patient is positioned supine with the arms out at 90° on secure arm boards. Quick-acting muscle relaxation is used for the procedure. The operating room bed is positioned with enough space in front of the anesthesia machine to allow the surgeon to conduct most of the procedure standing above the patient’ shoulder on each side. The patient is then prepped and draped in a sterile fashion, and Tegaderm™ dressings are applied to cover the nipple areolar complexes before the start of the procedure. All additional equipment, including the suction, cautery, and endoscopic tower, is positioned at the foot of the bed to allow free transition between the sides of the patient ( Fig. 2 A).
Instrumentation for the procedure includes a needle-tip electrocautery, a 4-prong skin hook, blunt-tip facelift scissors, a 25-mm (1-inch) fiberoptic retractor, two 25-mm Deaver retractors, and a pair of mirror-image Agris-Dingman dissectors (see Fig. 2 B). The basic endoscopic setup is that described by Price and colleagues, and consists of a 10-mm 30° angled endoscope with matching endoscopic retractor with combination suction cautery handle. The cautery is foot switched (Snowden-Pencer™/Cardinal Health- Dublin, OH). Although there are numerous cautery rod shapes available, the author prefers the J shape with the curve of the J oriented laterally on each side. The rods are designed so that the suction opening is above the spatulated cautery tip. The endoscopic tower and xenon light source are those that are routinely available in most surgery centers and hospitals.
Procedure
Incision and Initial Dissection
The incision is made in the axillary apex per preoperative marking, using a scalpel to the deep dermis. The incision is then completed into the subcutaneous plane using a needle-tipped electrocautery in cut mode ( Fig. 3 A). Dissection is then performed in the immediate subcutaneous plane, with a thin skin flap created in the direction of the lateral border of the pectoralis major muscle. A 4-prong skin hook is used to retract, with the correct thickness of the skin flap confirmed repeatedly during the dissection. This technique prevents damage to the axillary contents, the intercostobrachial nerve, and the skin flap (see Fig. 3 B). The 4-prong skin hook is then replaced as the dissection proceeds anteriorly, and is replaced with a fiberoptic retractor 25 mm (1 inch) wide. This retractor is used to identify the lateral edge of the pectoralis major muscle. Once the lateral muscle edge has been identified, the subpectoral space is entered under direct vision (see Fig. 3 C). A clean tissue plane is identified and developed between the pectoralis major muscle above and the pectoralis minor muscle below. The author prefers a finger-sweep–type approach to enlarge the initial area of the subpectoral space, with hemostasis then carefully confirmed with the aid of the fiberoptic retractor. Alternatively, this can all be done sharply, but the author has noted little advantage in this area. The key point is that the initial tissue dissection is performed to minimize blood staining to the tissues of the subpectoral space.