Never
Rarely
Sometimes
Usually
Always
Solid
0
1
2
3
4
Liquid
0
1
2
3
4
Gas
0
1
2
3
4
Wears pads
0
1
2
3
4
Lifestyle alteration
0
1
2
3
4
Generally, patients who are good candidates for muscle transposition procedures fit into one of several groups: (1) they have already failed one or more attempts at overlapping sphincteroplasty, (2) they have a specific congenital defect (e.g., spina bifida or imperforate anus) or an acquired defect (e.g., after trauma) that has left them without a sufficient sphincter complex, or (3) they have undergone a surgical exenteration (e.g., abdominoperineal resection). When (and if) a muscle transposition is entertained certainly depends on the skills and experience of the treating surgeon and the motivation of the patient. Before proceeding with a muscle transposition, any sources of pelvic/anorectal sepsis should be definitively controlled. The use of fecal diversion is discretionary but generally advisable. Plastic surgical consultation for the mobilization of the donor muscle may be important, depending on the skill set of the primary surgeon(s).
Graciloplasty
Gracilis Anatomy and Physiology
The gracilis muscle is a thin band present at the most medial portion of the thigh, with an average length of 41 cm and mean diameters of 4.4 cm (anteroposterior) and 1.1 cm (coronal) [7]. Proximally, it is attached to the pubic ramus, and distally it inserts on the proximal tibia. Its functional importance as an adductor is minimal. Innervation is through the obturator nerve (L2-3) and the muscle receives its blood supply via the circumflex femoral artery. Its relative lack of functional significance makes it an ideal candidate for surgical procedures in which transposition is required, and it can be mobilized as either a pedicled or a free flap. Gracilis transposition for incontinence was first described by Pickrell et al. [8] in 1952 and was performed in four children with spina bifida, with excellent reported success. The procedure has evolved over the past half century since its initial description, and we discuss the current state of technology regarding this procedure.
Procedure
As implied, graciloplasty involves mobilizing the gracilis muscle from its usual location into a circumanal position. Mobilization of the muscle is performed using either several small longitudinal incisions or a single longer incision, and great care is taken to preserve the major proximal blood supply and innervation. The tendon of the muscle is disconnected distally just below the knee. Counterincisions then are made on either side of the anal canal and the soft tissues around the anal canal are dissected to allow for the passage of the muscular loop through the skin of the upper thigh (on the side of the muscle donation) and around the upper anal canal. Based on the neurovascular supply of the gracilis muscle, endoscopically assisted gracilis muscle harvest has been described [9, 10]: a small transverse preliminary incision is made proximal to the knee to identify the gracilis tendon, and the endoscopic port permits a retrograde subfascial dissection through to a proximal medial thigh incision. This effectively results in reduced scar length and morbidity. The stimulating electrode (if used) is then placed distally (inferiorly, on the basis of the original muscular orientation) to the neurovascular bundle, and the electrodes are passed subcutaneously to the side of the implanted stimulator. The specific configuration of this loop depends on the patient’s anatomy and extent of scarring, but it may take an alpha, gamma, or epsilon loop configuration. With an alpha loop, the gracilis muscle is oriented as a sling (i.e., it does not make a complete loop around the rectum) and then is fixed at its distal tip to the ipsilateral ischial tuberosity. With the gamma and epsilon loops, the gracilis completely encircles the rectum and is fixed distally to the contralateral ischial tuberosity. With the gamma loop (shown in Fig. 32.1), the muscle is passed initially anteriorly, then posteriorly, whereas an epsilon loop initially passes the muscle posteriorly.
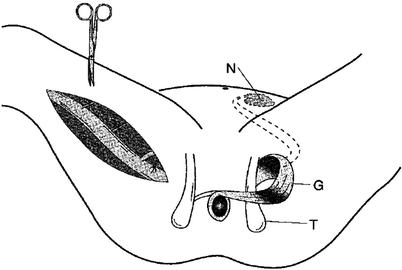

Fig. 32.1
Mobilization of the right gracilis muscle by ligation of the minor vascular pedicles. The major vascular pedicle and the obturator nerve with its branches are located proximally. On the left side, the dynamic graciloplasty configuration is shown after transposition of the muscle and implantation of the stimulation devices. N neurostimulator, G gracilis muscle, T ischial spine (Reprinted with permission from Chapman et al. [11])
Outcomes with Dynamic Graciloplasty
The literature to date regarding the success of dynamic graciloplasty is significant but not extensive. Chapman et al. [11] published a systematic review in 2002, analyzing outcomes reported in 17 original studies (383 patients) performed as of that time. Patients in the analyzed studies reported a success (continence) rate of 42–85 %. Other outcomes included measurements of pre- and postoperative anal canal pressures as well as the patients’ ability to delay bowel movements. Improvements were uniformly documented in these parameters as well as in quality of life measures. This study was rigorous in its approach and found that interpretation of data regarding patient outcomes was severely limited by variation in how outcomes were reported across the different studies.
Several studies published since this review by Chapman and colleagues [11] are worth mentioning. The largest study performed to date regarding the success of dynamic graciloplasty was published by Wexner et al. [12] in 2002, reporting a multi-institutional international trial that included 115 patients. Their study included patients with and without stomas at the time of the procedure. At a 2-year follow-up, patients without a stoma experienced a 55 % success rate (defined as a greater than 50 % reduction in incontinent episodes). Significant improvements were seen when comparing quality of life and activity inventories as assessed postoperatively versus preoperative determinations. Thornton et al. [13] reported results obtained when treating 38 patients with dynamic graciloplasty and found that both continence and satisfaction were good among patients with a functioning transposition. Despite significant expertise with the procedure, long-term outcomes were problematic, and at 5 years after the procedure only 16 % of patients were continent to solid stool. Penninckx [14] described the Belgian experience with 60 patients undergoing dynamic graciloplasty for acquired or congenital incontinence. Of these 60 patients, failure was noted in 27 (45 %).
The successes achieved in these patients are not without costs or complications. Chapman et al. [11] reported a 4 % mortality rate and a mixed morbidity rate of more than 100 %. The distribution of these complications is presented in Table 32.2. In the report of their multi-institutional experience, Wexner et al. [12] failed to report the complication rate, and longer-term follow-up with this large body of patients has not been published to date. In the Belgian study by Penninckx [14], the 60 patients experienced 61 complications requiring general anesthesia; however, there were no mortalities. Thornton et al. [13] also found approximately one significant morbidity per patient, with no mortalities.
Table 32.2
Complications with dynamic graciloplasty (n = 383)
Infection | 106 (28 %) |
Stimulator and lead faults | 59 (15 %) |
Leg pain | 54 (13 %) |
Gracilis or colon injury | 31 (8 %) |
Battery exhaustion | 29 (8 %) |
Constipation | 28 (7 %) |
Anal pain | 30 (7 %) |
Body pain | 24 (6 %) |
Stimulator-related pain | 15 (4 %) |
Neoanal stricture or stenosis | 16 (4 %) |
Deep venous thrombosis | 6 (2 %) |
Tendon displacement or detachment | 9 (2 %) |
Ineffective or noncontracting gracilis | 6 (2 %) |
Pulmonary embolism | 2 (1 %) |
Leg swelling, hematoma, seroma | 3 (1 %) |
Neuropraxia | 2 (1 %) |
Colonic fistula | 3 (1 %) |
For patients with poor function (either incontinence or constipation after a dynamic graciloplasty), either retrograde (transanal) or antegrade (via a colostomy/cecostomy or appendicostomy) colonic irrigation is a reasonable mechanism by which to salvage function. In this respect, Koch et al. [15] reported their experience with 46 patients (42 retrograde and 4 antegrade) who used colonic irrigation after graciloplasty and found a satisfaction rate of 81 %. Ho and Seow-Choen [16] also reported the successful use of neorectal irrigation as an adjunctive measure in patients with dynamic graciloplasty, primarily those who had problems with evacuation. Saunders et al. [17] have reported a specific body of experience with antegrade enemas via colonic conduits in 14 patients, and the majority of patients (57 %) were able to be managed successfully using this technique.
The relatively high burden of morbidity relative to the modest rates of efficacy naturally raises the discussion of patient selection. Unfortunately, the studies performed to date were not sufficiently powered to delineate which specific patient characteristics were associated with the likelihood of success versus failure. The study performed by Wexner et al. [12] examined the relationship between duration of symptoms and outcomes but failed to show any workable relationship. In the absence of clear guidance, there are several principles that should direct the use of this procedure. First, patients should be fit and able to tolerate complication(s) and the distinct possibility of a reoperation/revision. Second, they should be highly motivated and counseled extensively regarding the risks and complications of the procedure as well as its alternatives.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








