Category
Recommended follow-up
Negative
Regular 1 year
Indefinite
Probably negative
Regular 1 year
Unknown
Short interval
Probably positive
Short interval
Positive
Low grade
Short interval or colectomy if there is a mass
High grade
Colectomy
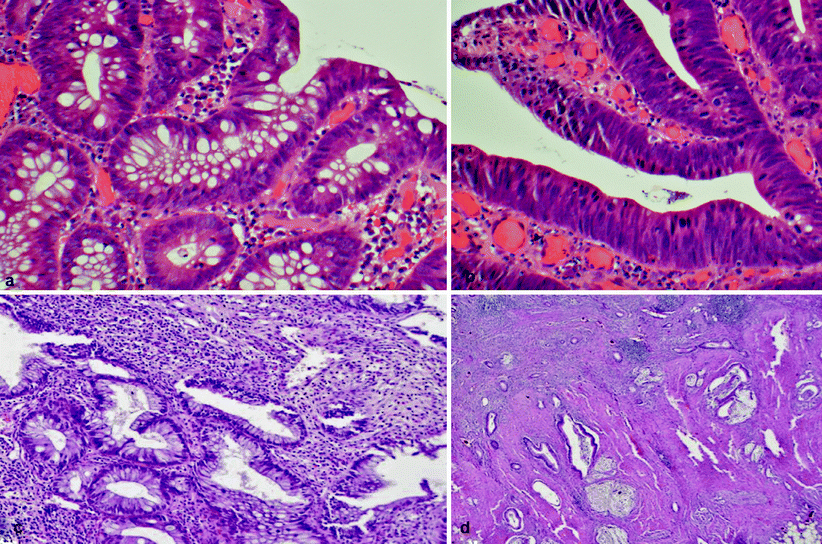
Fig. 19.1
(a) Low grade dysplasia with uniform, elongated, crowded, basally located nuclei and a few mucin vacuoles. (b) High-grade dysplasia with marked nuclear stratification, loss of cellular polarity, and abundant mitoses. (c, d) Low-grade tubuloglandular adenocarcinoma with well-differentiated adenocarcinoma (d) arising from low-grade dysplasia (c). The invasive glands show minimal atypia with little or no desmoplasia
1.
LGD (see Fig. 19.1a) is a lesion characterized by crowded glands that vary slightly in size, similar to colonic tubular adenomas. These glands are populated by dysplastic cells with large, spindle-shaped, basally orientated hyperchromatic nuclei. There is little or no inflammation.
2.
HGD (see Fig. 19.1b) is associated with major architectural abnormalities of the glands, which are lined by large vesicular stratified nuclei, prominent nucleoli with or without loss of orientation, and abnormal mitoses. There is little or no inflammation.
The original classification of dysplasia created by Riddell et al. remains essentially unchanged. In 2000, a group of gastrointestinal pathologists from Europe, Japan, and North America gathered in Vienna, Austria, to propose a new classification to bridge the gap between the terminologies used by these various groups (Table 19.2) [49, 50]. However, there are no important differences between the original classification and that created in 2000 with regard to patient management, and we recommend using both systems when needed.
1. Negative for dysplasia/neoplasia |
2. Indefinite for dysplasia/neoplasia |
3. Noninvasive, low-grade neoplasia (low-grade adenoma/dysplasia) |
4. Noninvasive, high-grade neoplasia (high-grade adenoma/dysplasia, noninvasive carcinoma, suspicious for invasion) |
5. Invasive neoplasia (intramucosal carcinoma, submucosal carcinoma or beyond)a |
When IND is detected during initial colonoscopy, progression to advanced neoplasia occurs in approximately 13–26 % of cases [9, 17]. In a study from Mount Sinai Hospital in New York, the 5-year rate of progression to either HGD or CRC was 1.1, 9, and 45 %, respectively, in patients who initially had no dysplasia, a status of IND, and a status of LGD [51]. A review of ten prospective studies reported that when LGD was found during initial surveillance colonoscopy, HGD or CRC developed in up to 29 % of patients [17].
Interobserver Variation in the Interpretation of Dysplasia
There is a significant degree of inter- and intraobserver variability in the histological diagnosis of dysplasia. Collins et al. [52] found that agreement is best when identifying HGD but is less accurate for IND and LGD. This is, in part, due to variation in the understanding of the term indefinite for dysplasia, a label usually given to specimens that are inadequate in size or orientation or when there is severe inflammation and secondary reactive change [24]. In this regard, less experienced pathologists tend to use IND more than experienced pathologists. Nevertheless, even amongst experts, variation occurs and overall agreement is less than optimal [53]. This has led to the recommendation that two pathologists, one of whom is an experienced gastrointestinal pathologist, should report these cases of dysplasia by consensus [54].
Recently, the positive presence of alpha-methylacyl-coenzyme A racemase, a peptide expressed in a high proportion of prostatic intraepithelial neoplasia and prostatic carcinomas, has not been expressed in any UC foci that are considered negative for dysplasia, but it has been shown to be significantly increased in foci of LGD (96 %), HGD (80 %), and adenocarcinoma (71 %) [55]. This immunostain has been used as an adjunct in difficult cases.
Number of Biopsies Required to Detect Dysplasia
A second controversial point is the number of biopsies required for an accurate diagnosis or exclusion of dysplasia. Itzkowitz and Harpaz [15] commented that a typical biopsy samples less that 0.05 % of the total colonic surface area. It is, therefore, universally accepted that a considerable number of biopsies should be taken, but there is wide variation in current practice. Rubin et al. [56] showed that at least 33 biopsies are required to detect dysplasia with 90 % accuracy and that 64 biopsies would be required to increase this accuracy to 95 %. The Crohn’s and Colitis Foundation of America [23], the American College of Gastroenterology [57], and the British Society of Gastroenterology [58] all differ in their recommendations of the exact number of biopsies needed for accurate detection of dysplasia and the technique required to obtain this. However, in practice, the number of biopsies taken is often less than that indicated in any of the recommended guidelines [59, 60]. The practical recommendation is to take four random biopsies every 10 cm along the colon, and extra biopsies should be obtained from any strictured or abnormal areas. In patients with UC, it is recommended that four-quadrant biopsies be taken every 5 cm in the distal sigmoid colon and the rectum [23, 58]. Full colonoscopy is necessary because approximately one third of UC-associated CRC develops in the proximal colon [27, 61].
New Endoscopic Techniques in the Detection of Dysplasia
Improving the detection of dysplastic lesions in patients with IBD should reduce sampling errors during colonoscopy, thereby improving overall efficiency. This can be enhanced by targeted rather than random colonic biopsies [27]. Two such developments are spraying dye in the mucosa (chromoendoscopy) and the use of a high-resolution colonoscope. Chromoendoscopy detects a significantly higher number of neoplastic lesions [62, 63] but does not significantly increase examination performance time. The new guidelines of the British Society of Gastroenterology [61] recommend that pancolonic dye spraying be adopted by endoscopists as the technique of choice. If chromoendoscopy is not used, the strategy outlined in the 2002 guidelines should be followed (namely, two to four random biopsies from every 10 cm along the colon) [58, 61]. The use of chromoendoscopy by appropriately trained endoscopists was formally endorsed by the American Gastroenterological Association [27]. With chromoendoscopy, the crypt architecture can be categorized by evaluation of the pit pattern, which facilitates differentiation between neoplastic and nonneoplastic changes and enables the performance of targeted biopsies [27]. Moreover, chromoendoscopy has been shown to provide a more accurate evaluation of the extent of disease and the degree of inflammatory activity [63].
Another promising area is the use of confocal laser endomicroscopy, which permits in vivo histology during endoscopy and, when combined with chromoendoscopy, significantly decreases the number of biopsies required for cancer surveillance in patients with UC, providing a fourfold higher diagnostic yield compared with white-light endoscopy used for random biopsies [64].
Molecular Pathology of CRC in IBD
A number of differences exist between the molecular pathology of sporadic and colitis-associated CRC. Loss of function of the adenomatous polyposis coli gene is a common and early event in sporadic CRC, but it is much less frequent in UC and usually occurs late in the dysplasia-carcinoma sequence of colitis-related CRC [15]. In contrast, p53 mutations arise late in sporadic CRC, whereas in IBD they occur early and seem to play an important role. In this respect, Burmer et al. [64] found that p53 loss of heterozygosity correlates with malignant transformation and occurs in 9 % of biopsy specimens of those cases labeled as IND, 33 % with LGD, 63 % with HGD, and 85 % with CRC [65]. Mutations of the DPC4 and K-ras genes are common events in sporadic CRC but are infrequent in patients with IBD and cancer [66, 67]. Finally, Schulmann et al. [68] found that the frequency and profiles of high-level microsatellite mutations differ significantly between sporadic and colitic CRC. It is anticipated that these molecular biologic characteristics will provide new profiles for risk categorization in IBD-related CRC and will continue to be a principal area of future research [69].
Management of Dysplasia
The management of patients with dysplasia (Fig. 19.2) has been the subject of a controversial debate for many years. Dysplasia is grossly divided into flat (endoscopically undetectable) or raised (endoscopically detectable). For patients who test negative for dysplasia, regular follow-up and endoscopic surveillance should continue per national guidelines. In cases of IND due to inflammation, patients should undergo treatment for colitis, allowing the inflammation to subside before biopsy is repeated. If there is still a nuclear abnormality, then changing the diagnosis to flat LGD is warranted. If the grade remains classified as IND, then closer follow-up and repeat biopsies should be performed.
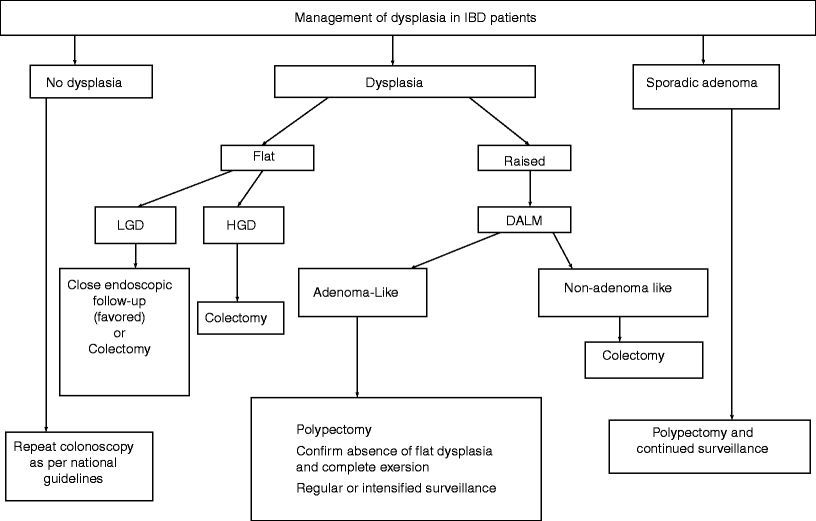

Fig. 19.2
The management of dysplasia in patients with irritable bowel disease (IBD). LGD low-grade dysplasia, HGD high-grade dysplasia, DALM dysplasia-associated lesion or mass. (Modified from reference [24])
Flat Dysplasia
The management of flat LGD is controversial. A number of studies have reported a 50 % rate of progression from LGD to HGD or CRC over 5 years [19, 53, 70]. In patients who have undergone urgent colectomy for LGD, coexisting CRC has been found in 16–34 % of specimens [17, 70]. In this regard, Ullman and colleagues [70] found the rate of progression to be independent of focality because unifocal and multifocal flat LGD progressed at similar rates. This group also reported that the presence of flat LGD was a “powerful predictor of advanced neoplasia” and that continued surveillance as opposed to immediate colectomy was “at best a risky strategy.” The 30-year experience of St. Mark’s Hospital, London, also found similar results: coexisting CRC was found in 20 % of patients with LGD who were undergoing colectomy, and 39.1 % of patients with LGD progressed to HGD or CRC during follow-up [9].
However, there are other schools of thought concerning this issue. Lim et al. [71] showed that only 10 % of patients with LGD developed HGD or CRC compared with 4 % of patients with UC without dysplasia after 10 years of follow-up. They concluded that the diagnosis of LGD is insufficient to justify colectomy. Similar findings have been reported by Befrits and colleagues [72]. We approach these cases through discussion during multidisciplinary team meetings involving gastroenterologists, pathologists, and colorectal surgeons; a combined decision should be made and patients should be informed about the possibility or probability of negative colectomy and the potential morbidity and complications associated with surgical treatment versus a wait-and-see policy. If colectomy is not performed, then close endoscopic and clinical follow-up should be continued [24].
There is little controversy surrounding flat HGD because there is concurrent CRC in 42–45.5 % of colectomy specimens from these patients [9, 17, 73]. Therefore, there is a general agreement that the presence of HGD warrants prompt colectomy. LGD in a mass lesion [74] that does not resemble a typical sporadic adenoma and cannot be resected endoscopically, or a stricture that is symptomatic or is not passable during colonoscopy [35, 36, 75], especially in longstanding disease, often is indicative of coexistent colon cancer, necessitating a colectomy.
A recent publication in the pathology literature identified low-grade tubuloglandular adenocarcinoma (see Fig. 19.1c, d) as a finding responsible for 11 % of IBD-associated adenocarcinomas, and superficial LGD was associated with underlying invasive adenocarcinoma [14]. This finding has reinforced the recommendation of colectomy for patients with LGD. A study by Rodriguez et al. [73] showed that 60 % of gastroenterologists in the US did not recommend immediate colectomy for a confirmed finding of LGD but rather favored continued and closer surveillance [27]. Interestingly, recent work by Siegel and colleagues [76] has shown that almost all patients who responded to questionnaires in the study recognized that UC raises their chance of getting colon cancer. In this study, 60 % of patients would refuse a physician’s recommendation for elective colectomy if dysplasia was detected, despite being told that they had a 20 % risk of having cancer at the time. On average, these patients would agree to colectomy only if their risk of colon cancer at that time were at least 73 %.
Epithelial regeneration and repair, especially in the setting of active inflammation, may result in atypia, which can be difficult to distinguish from true dysplasia. These cases are classified as IND [77]. Patients whose biopsy specimens are IND are a poorly studied group of patients with IBD. In a 1991 report by Nugent et al. [78], 20 patients with biopsies considered “indefinite” were followed for an unspecified length of time. Three subjects developed HGD, one of whom was discovered to have an adenocarcinoma at the time of colectomy.
Raised Dysplasia
Any raised lesion within an area affected by colitis is referred to as a dysplasia-associated lesion or mass (DALM); this first was described in 1981 by Blackstone et al. [74]. In their original report, a high incidence of CRC in these lesions was found. Other studies showed the rates of CRC in the presence of DALMs to be between 31 and 65 % [9, 17, 52]. As a result, initially the presence of a DALM posed a high risk for either concurrent CRC or progression to CRC, and colectomy was strongly recommended. In recent years, studies have demonstrated that not all polypoid lesions in patients with IBD necessitate radical treatment in the form of colectomy. Separation of DALM from sporadic adenoma posed another problem because the former required colectomy whereas the latter can be managed adequately with polypectomy and subsequent surveillance [79].
Studies have evaluated the value of immunohistochemistry in separating DALM from sporadic adenoma. Neither histological appearance nor immunohistochemistry has been shown to be useful in clinical practice. These differences are now of no clinical value in the treatment of patients harboring these lesions. Recent evidence shows that dysplastic masses detected outside of an area of colitis may be reliably diagnosed as sporadic adenomas and treated as such [80, 81].
Recent studies have shown that DALM can be classified grossly into those that endoscopically resemble sporadic adenomas – and hence are called adenoma-like DALMs (ALMs) – and those that do not look like adenoma and hence are labeled non-adenoma-like DALMs (NALDs) [82–84]. ALM endoscopically appears as a well-circumscribed, smooth or papillary, nonnecrotic, sessile or pedunculated polyp [82, 85], whereas NALD lesions appear as velvety patches, plaques, irregular bumps or nodules, or stricturing lesions that are not amenable to endoscopic resection [86]. Recent reports [82, 83] have shown that ALM lesions can be treated safely with endoscopic excision, provided that they are completely excised and that multiple biopsies are taken from the base of the stalk and the adjacent flat mucosa after excision, each of which must show no evidence of dysplasia. If such endoscopic excision is performed, close follow-up surveillance should be carried out in case further dysplastic lesions develop. This is supported by the fact that there were no significant differences in the incidence of polyp formation during follow-up after the initial resection between patients with UC and an ALM (62.5 %) and patients with UC and a known sporadic adenoma (50 %) or between either of these two UC patient subgroups and a control group of patients with no UC sporadic adenoma (49 %) [84].
It is important to realize, however, that the differentiation of ALM, NALD, and inflammatory polyps should be done endoscopically, and this can be a challenging task. A recent article from Farraye et al. [87] showed conclusively that even expert gastroenterologists have difficulty differentiating these polyps. It is also worth noting that Rutter et al. [33], while looking retrospectively at the cases from St. Mark’s Hospital, found that most dysplasia was raised and fell into the ALM category. In this regard, this same group evaluated 56 patients with UC who developed dysplasia (either flat or raised) in the course of a 14-year surveillance program conducted at St Mark’s Hospital in London [86]. Here, a total of 110 neoplastic areas were detected in the 56 patients, of which 77.3 % were visible during colonoscopy. More specifically, 74 of the visible lesions (87 %) were “polypoid” (adenoma-like), four were described as having an “irregular” outline, and one was described as a “plaque.” In addition, six were described as macroscopic cancers. There is high association of cancer, ranging from 38 to 83 %, with NALDs that are considered to be endoscopically unresectable. For this reason, it is recommended that patients with UC and an endoscopically unresectable NALD should undergo a colectomy, regardless of the grade of dysplasia detected on biopsy analysis [88]. These recommendations apply to patients with UC regardless of their age or duration and the extent of their colitis [82, 88]. Although a scar may be detectable after colonoscopic resection, it is advisable to tattoo the mucosa adjacent to any suspicious lesion that is resected endoscopically for ease of future identification. From a practical perspective, the important issue is to determine whether the lesion is completely resectable endoscopically without adjacent dysplasia and whether the rest of the colon is also free of dysplasia.
Surveillance in Patients With IBD
Because cancer develops through a chronic inflammation-dysplasia-cancer pathway in IBD, patients begin a surveillance program to detect dysplasia before the development of cancer [31]. The practice of surveillance, however, varies widely. In a recent questionnaire-based study by Rodriguez et al. [73], nearly 80 % of the responding physicians stated that they begin surveillance colonoscopy at 8–10 years of disease duration for patients with pancolitis and 54 % reported sending at least 31 biopsies. However, in a study from the Netherlands, surveillance was started correctly in patients with pancolitis by 53 % of respondents and in cases of left-sided colitis by 44 % of the gastroenterologists, and fewer than 30 biopsies per colonoscopy were taken by 73 % of the respondents [89]. Patient compliance with the surveillance program is also a problematic clinical issue and a confounding variable. Patients must be counseled about the risk of developing CRC and about the risk of developing cancer if they drop out of the surveillance program. They also should be informed that cancer may develop without previous detection of dysplasia [15].
Evidence for Surveillance in Ulcerative Colitis
Morson and Pang reported the coexistence of cancer in colectomy specimens in five of nine patients with UC who already had received diagnoses of HGD from previous preoperative rectal biopsies [90]. This led to the development of surveillance programs using multiple biopsies to detect dysplasia. St. Mark’s Hospital was the first in the world to instigate such a colonoscopic surveillance program in patients with UC [91]. Since then, the practice of surveillance has become widely adopted, although the evidence of its benefits is still somewhat limited. Rutter et al. [9] reported a 30-year experience involving 600 patients with longstanding, extensive UC, and they concluded that their study “shows that colitis surveillance can be effective.” At present, no randomized studies have documented a reduction in the risk of developing, or dying from, CRC through the systematic use of surveillance colonoscopy. Limited evidence is available from studies examining the effectiveness of surveillance versus no surveillance, largely because of ethical issues given the available interpretable data.
In a Cochrane analysis of three studies [92–94], 8 of 110 patients in the surveillance group died of CRC compared with 13 of 117 patients in the nonsurveillance group [95]. The Cochrane analysis concluded the following: There is evidence that cancers tend to be detected at an earlier stage in patients who are undergoing surveillance and these patients have a correspondingly better prognosis, … There is indirect evidence that surveillance is likely to be effective in reducing the risk of death from IBD-associated cancer.” A fourth recent study by Lutgens et al. [96] showed a significant difference in 5-year cancer-related mortality rates in people undergoing surveillance compared with a no surveillance policy.
Evidence for Surveillance in Crohn’s Disease
There are few data about the effectiveness of surveillance in Crohn’s colitis. One study that followed 259 patients with extensive Crohn’s colitis (affecting at least one third of the colon) found that 7 % of patients had dysplasia or cancer upon screening colonoscopy, and an additional 14 % had dysplasia or cancer found during surveillance examinations [97]. In another study by Friedman and colleagues [98




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








