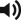Introduction
The superior gluteal myocutaneous free flap was first described by Fujino in 1975 for breast reconstruction. In 1978, Le Quang performed the first breast reconstruction with an inferior gluteal myocutaneous free flap. Shaw popularized the myocutaneous superior gluteal artery free flap; however, a short vascular pedicle often led to additional vein grafting, thus decreasing enthusiasm. The inferior gluteal myocutaneous flap championed by Paletta et al. was also abandoned due to sciatic nerve injury.
In 1993, the superior gluteal artery perforator (SGAP) flap was first introduced by the authors’ group. This fasciocutaneous flap utilizes the upper buttock adipose tissue and overlying skin leaving the underlying gluteus maximus muscle intact. However, the donor site is at risk for a bowing contour deformity, disrupting the superior fullness of the upper buttock. In early 2004, we developed the inferior gluteal artery perforator (IGAP) flap. This flap uses excess lower buttock tissue, preserves the underlying muscle, and leaves the scar in the natural depression of the inferior gluteal crease resulting in a more desirable donor site.
Free flaps based on the gluteal artery perforators are an excellent alternative for breast reconstruction in patients with inadequate abdominal tissue, a history of extensive abdominal liposuction, prior abdominoplasty, or otherwise unavailable abdominal donor tissue. These flaps can also be used to supplement deep inferior epigastric perforator (DIEP) flaps in a stacked flap fashion in women with large breasts desiring large reconstruction who have insufficient donor tissue from any one site alone. While the profunda artery perforator flap is the first alternative to the DIEP flap in our practice, the SGAP and IGAP flaps remain superb options. The decision between a SGAP or an IGAP flap is primarily based on the patients’ target breast volume, their anatomic distribution of adipose, quality and location of perforators, and their scar preference. Gluteal artery perforator (GAP) flaps are also a common choice for local coverage of pressure ulcers because of their bulk, minimal donor site morbidity, and adaptability of the flap design based on perforators available and the defect present. Customizing a posterior thigh fasciocutaneous flap based on the descending branch of the inferior gluteal artery is an option for pelvic or perineal reconstruction after cancer ablation, trauma, or congenital defects.
Flap Anatomy ( Figs 58.1 , 58.2 and Fig 13.3 , Fig 13.19 )
Arterial and Venous Supply of the Flap ( Fig. 58.2 , see Fig. 13.3 )
SGAP
Dominant:
superior gluteal artery
Length: 7 cm (range 5–10 cm)
Diameter: Range 2.0–3.0 mm
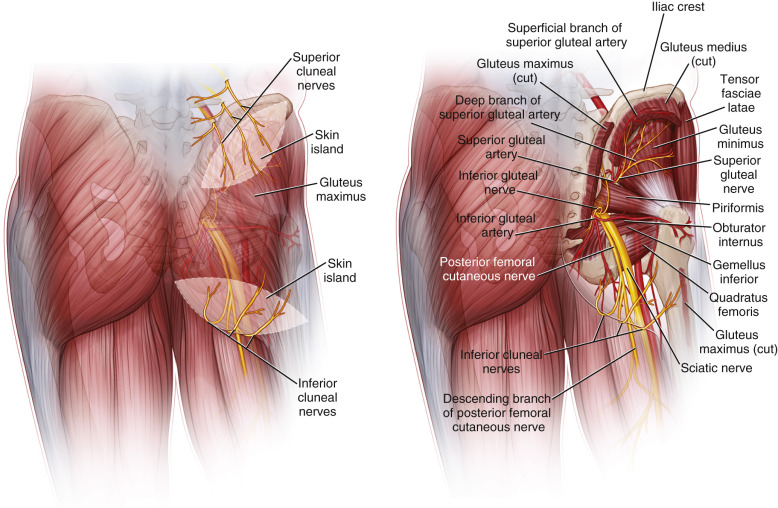
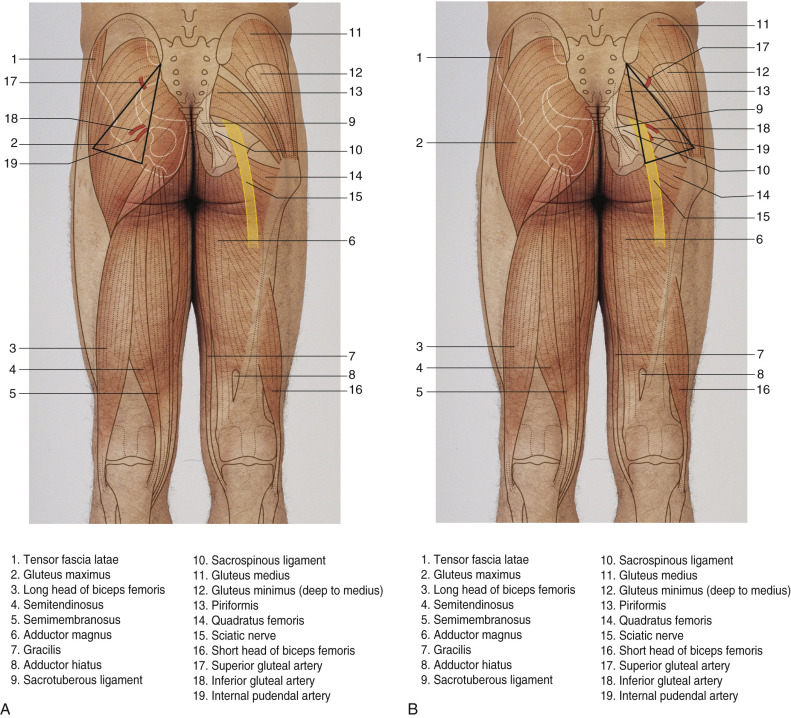
The superior gluteal artery, the largest branch of the internal iliac artery, is a continuation of the posterior division of that vessel. It is a relatively short artery, which runs dorsally between the lumbosacral trunk and the first sacral nerve. It emanates from the pelvis above the upper border of the piriformis muscle, where it immediately divides into both superficial and deep branches. The superficial branch continues on to supply the upper portion of the gluteus muscle and overlying fat and skin. Topographic landmarks can be used to identify the superior gluteal artery ( Fig. 58.3 ). With the femur slightly flexed and rotated inward, a line is drawn from the posterior superior iliac spine to the posterior superior angle of the greater trochanter. The point of emergence of the superior gluteal artery from the upper part of the greater sciatic foramen corresponds to the junction of the upper and middle thirds of this line. Perforating vessels are given off from the superior branch of the SGA. An average of three perforators can be found to supply the skin .
IGAP
Dominant:
inferior gluteal artery
Length: 7.5 cm (range 6–9 cm)
Diameter: Range 2–2.5 mm
The inferior gluteal artery is a terminal branch of the anterior division of the internal iliac artery and exits the pelvis through the greater sciatic foramen. Landmarks can also be used to identify the location of the emergence of the inferior gluteal artery outside the pelvis. A second line is drawn from the posterior superior iliac spine to the outer part of the ischial tuberosity; the junction of its lower-third with its middle-third marks the point of emergence of the inferior gluteal and its surrounding vessels from the lower part of the greater sciatic foramen. The artery accompanies the greater sciatic nerve, internal pudendal vessels, and the posterior femoral cutaneous nerve. In this subfascial recess, the inferior gluteal vein will receive tributaries from other pelvic veins. The inferior gluteal vasculature continues towards the surface by perforating the sacral fascia. It exits the pelvis caudal to the piriformis muscle. Once under the inferior portion of the gluteus maximus, perforating vessels are seen branching out through the substance of the muscle to feed the overlying skin and fat. The course of the inferior gluteal artery perforating vessels is more oblique through the substance of the gluteus maximus muscle than the course of the superior gluteal artery perforators, which tend to travel more directly to the superficial tissue up through the muscle. Thus, the length of the inferior gluteal artery perforator and the resultant pedicle length for the overlying IGAP flap are greater than that found with an SGAP flap. Because the skin island is placed inferior to the origin of the inferior gluteal vessels, a longer pedicle is also assured.
The direction of the perforating vessels can be superior, lateral, or inferior. Perforating vessels that nourish the medial and inferior portions of the buttock have relatively short intramuscular lengths (4–5 cm), depending on the thickness of the muscle. Perforators that nourish the lateral portions of the overlying skin paddle are observed traveling through the muscle substance in an oblique manner 4–6 cm before turning upwards towards the skin surface. By traveling through the muscle for relatively long distances, these vessels are much longer than their medially based counterparts. The perforating vessels can be separated from the underlying gluteus maximus muscle and fascia and traced down to the parent vessel, forming the basis for the inferior gluteal artery perforator flap. Between two and four perforating vessels originating from the inferior gluteal artery will be located in the lower half of the gluteus maximus .
After giving off perforators in the buttocks, the inferior gluteal artery then descends into the thigh accompanied by the posterior femoral cutaneous nerve and follows a long course, eventually surfacing to supply the skin of the posterior thigh.
Minor:
none of clinical significance.
The gluteus maximus muscle is classified as a Mathes-Nahai Type III muscle based on its vascular anatomy with two major pedicles, the superior and inferior gluteal arteries, and no minor vessels. These arteries also supply the overlying skin and are the basis of these fasciocutaneous flaps.
Venous Drainage of the Flap (see Fig. 13.19 )
SGAP
Primary:
venous comitantes
Length: 7.5 cm (range 6–9 cm)
Diameter: Range 2.5–4.0 mm
Secondary:
there are no known clinically significant vessels that can be used to either solely drain the flap or to provide partial or augmented drainage.
IGAP
Primary:
venous comitantes
Diameter: Range 2–3 mm
Surgeon must be cognizant of recipient venous diameter when harvesting donor vessels. Internal mammary vein typically range at 2–4.5 mm but more commonly 2–2.5 mm.
Secondary:
there are no known clinically significant vessels that can be used to either solely drain the flap or to provide partial or augmented drainage
Flap Innervation ( Fig. 58.1 and Fig 13.3 , Fig 13.19 )
Sensory:
SGAP and IGAP flaps are not typically harvested as an innervated flap.
However, they can be harvested as sensate flaps when indicated based on: superior clunial nerves (branches of posterior rami of lumbar spinal nerves L1-L3), medial clunial nerves (arise from the posterior rami of sacral spinal nerves S1-S3), or inferior cluneal nerves (gluteal branches of the posterior femoral cutaneous nerve); direct branches off the spinal cord L1–S3.
Motor:
not harvested with motor innervation.
Flap Components
The SGAP and IGAP flaps can be designed and harvested in variable ways to accommodate different defects and goals. When harvested as free flaps (e.g., breast reconstruction) both the SGAP and IGAP flaps are harvested as fasciocutaneous flaps (skin, fat, fascia). They can also be designed as myocutaneous advancement flaps, incorporating the gluteus maximus muscle in addition to the fascia, fat, and skin (e.g., sacral and ischial wounds). (See also Ch. 44 .)
Advantages
- •
Both : Great options for designing a pedicled perforator flap for coverage of decubiti and lower back defects because of their consistently adequate tissue volume and vasculature. Their adaptable design can accommodate a large range of local defects while retaining potential for primary closure without need for skin grafting. They are good alternative methods for breast reconstruction when abdominal or posterior thigh donor sites are unavailable or inadequate, especially with a patients’ pear-shaped body habitus ( Table 58.1 ). Unlike in some autologous tissue alternatives, the tissue available is usually adequate for breast reconstruction without an implant. Even very thin women often have sufficient tissue for breast reconstruction. The fat from this location has a firm consistency contributing to breast projection. The flaps can be rotated to replicate the patient’s natural footprint and conus. Both have minimal donor site morbidity and with no muscle is sacrificed and eliminate the risk of hernia or abdominal bulge.
Table 58.1
The gluteal region as a donor site for breast reconstruction
Indication
(%)
Thin abdomen with inadequate tissue for desired breast size
64
Abdominal incisions
14
Previous abdominoplasty
8
Patient preference
7
Nulliparous
6
Failed abdominal flap
1
- •
SGAP has a thick flap with adequate pedicle for breast reconstruction. It is a short easy dissection.
- •
IGAP also has a thick flap with a long pedicle which, in breast reconstruction, often supersedes rib cartilage removal as the IMA recipient need not be as long. The long pedicle permits the option of use of the thoracodorsal system when it is indicated. IGAP is an optimal choice in patients with excessive saddle-bag tissue, utilizing this tissue as the donor results in an aesthetically pleasing body contour at the donor site. Preservation of the superior buttock fullness results in fewer contour defects than the SGAP and the scar can be hidden if done in the gluteal crease.
Disadvantages
- •
Both : When used for bilateral breast reconstruction these flaps require patient repositioning during surgery. They are typically inappropriate for most arm and head and neck reconstruction, because of their thickness. Patients should be aware that there is always the risk of donor site contour defects, visible scar, and asymmetry, and although more common following SGAP, there is also a risk after IGAP.
- •
SGAP : The short course through muscle makes for a technically simple procedure but the shorter pedicle limits its applicability. ( Note : perforators located laterally produce longer pedicles).
- •
IGAP : A discrepancy of the vein caliber is a potential problem when using internal mammary vessel as a recipient. Postoperative pain and discomfort are more common with the IGAP and it has an increased risk of wound dehiscence.
Preoperative Preparation
As with all surgeries, a complete history and physical exam are completed during the initial consultation. Any comorbidities are addressed, options and expected outcomes including scar location are discussed, and risks of surgery are explained. Patients should be informed about possible contour deformities and the future location of the scar at the buttock.
Preoperative imaging with computed tomographic or magnetic resonance imaging angiograms of the patient in the prone position is essential for optimal preoperative flap design. The angiograms identify the key perforator’s course as being musculocutaneous or septocutaneous, their location, and their caliber – thus, influencing the choice between a SGAP or IGAP flap and providing a map for preoperative planning.
Other influences on preoperative flap selection are the patient’s unique body habitus and distribution of fat, the extent of radiotherapy or trauma to the surrounding tissue when applicable, ambulatory status, and the tissue components that need to be replaced to reach the functional and structural goal of the reconstruction. The patient’s personal preference of scar location is another important component in flap choice.
For breast reconstruction, the patient is typically seen in our office the day before surgery for marking with a permanent marker and a final review of the surgical plan and discussion of any unanswered question. With the patient in an upright position, the midline of the chest and the inframammary fold are marked, as well as suggested mastectomy incision lines.
For all uses of these flaps, the perforator location identified through angiography is confirmed with a Doppler probe and marked. The flap design is marked and measured as described below.
Flap Design
Anatomic Landmarks ( Fig. 58.3 )
With the hip slightly flexed and rotated inward, a line is drawn from the posterior superior iliac spine to the posterior superior angle of the greater trochanter; the point of emergence of the superior gluteal artery corresponds to the junction of the upper- and middle-thirds of this line. Additional perforators may be located lateral to this line. These lateral perforators are septocutaneous, passing between the gluteus maximus and medius, and are reliably longer in length.
A second line is drawn from the posterior superior iliac spine to the outer part of the ischial tuberosity; the junction of the lower and middle-third marks the point of emergence of the inferior gluteal arteries from the lower part of the greater sciatic foramen.
General Thoughts About Flap Design
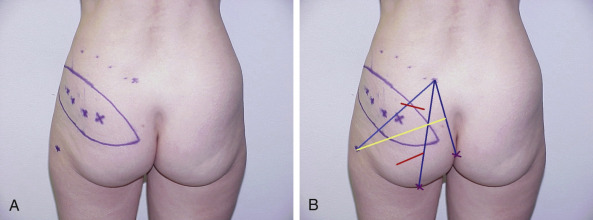
SGAP ( Fig. 58.4A,B )
Markings are placed on the patient in the lateral prone position.
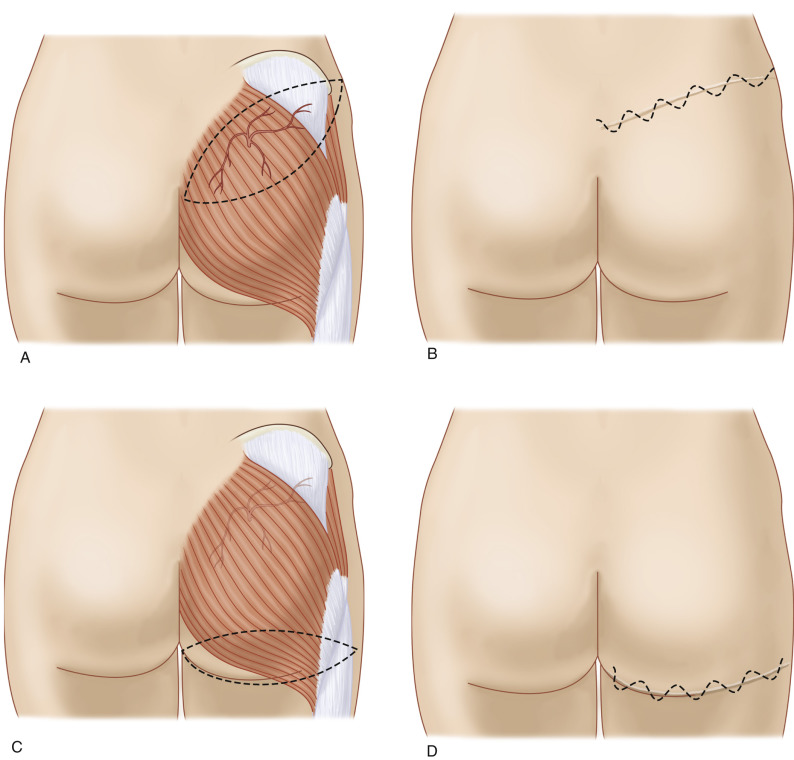
The skin paddle is marked, beginning at the most inferior and medial point, 2–3 cm below the intergluteal crease. It extends superiolaterally in an elliptical pattern, including the perforators and provide tissue adequate to correct the defect dimensions. The skin flap can be customized to almost any orientation as long as the outline contains a perforator. The scar of an oblique elliptical flap can be designed to be concealed by swimwear or undergarment. It should be noted that perforators located laterally will produce longer pedicles and should therefore be incorporated into the flap design when this characteristic is desirable ( Fig. 58.5A,B ).