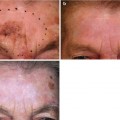
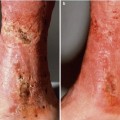
Fig. 6.1
Columella, ala rim and base of nostril SCC presenting a challenge to surgically repair without prosthesis
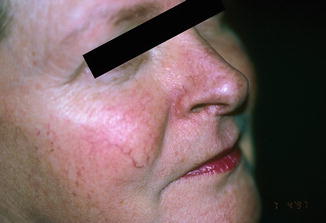
Fig. 6.2
Good cosmetic result 2 years following treatment
4.
Where radiotherapy may give a better (at least in the short term) cosmetic outcome, e.g. philtrum of upper lip and oral commissure (Figs. 6.3 and 6.4).
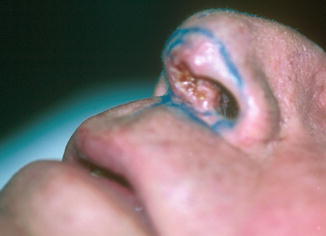
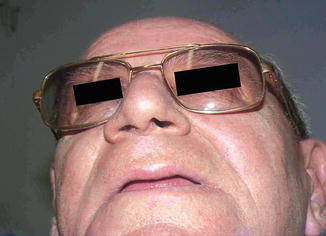

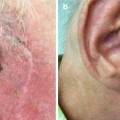
Fig. 6.3
Right ala nasi and philtrum of upper lip BCCs pretreatment

Fig. 6.4
Following completion of treatment, good cosmetic result not obtainable by surgery
5.
Where surgery may cause nerve damage or functional impairment, e.g. tumours overlying spinal accessory nerve or marginal mandibular nerve.
6.
Patients with deep or lateral marginal involvement following excision of tumours, where further surgery is not feasible, not likely to be tolerated or refused.
7.
Patients with high risk of microscopic residual disease, e.g. completely excised tumours with perineural invasion with no clinical signs of perineural invasion, or following curettage of poorly differentiated squamous cell carcinomas.
8.
Selected patients with small volume or marginal recurrent disease following surgery (in these cases, the area should include the full length of the surgical scar and a generous margin).
Opinions differ regarding suitability of morpheic BCC for superficial X-ray therapy. Although there may be a higher risk of recurrence with radiotherapy (as there is for all other forms of therapy) and reported cure rates vary [7], we will use radiotherapy for morpheic BCC selecting larger margins and more penetrating qualities.
We avoid treating middle third of the upper eyelid to avoid the risk of keratinisation of the palpebral conjunctiva and also avoid treating scrotal skin.
We generally insist on biopsy confirmation and if there is doubt to the extent of the lesion, then biopsies to assess the extent of tumour are taken.
Debulking tumours will reduce acute reactions and may allow the use of less penetrating X-ray qualities. We generally use the same doses for basal and squamous cell cancers.
Treatment on other areas of the body is usually only considered if surgery or other treatments are not feasible and should be avoided below the knee because of very slow healing times. (Multiple fractions or hyperfractionation should be considered if X-ray is used in poor healing sites.)
Patients should be over 50 years of age, not pregnant, able to give informed consent for treatment (acknowledging the risks of developing a radiation induced scar and the very low risk of future skin cancer development in the site) and be able to attend for fractionated treatment. This latter requirement is often the most difficult treatment-limiting step. They should obviously not have a contraindication to radiation treatment such as idiosyncratic reactions, sister chromatid exchange deficiency syndromes, Gorlin’s syndrome, ataxia telangiectasis or previously irradiated tumour.
Very large tumours or tumours with bone involvement, Merkel cell carcinoma, malignant sweat gland tumours and named nerve perineural invasion are all best treated at specialised oncology/radiation treatment centres and are beyond the scope of office radiotherapy [9].
Current opinion in Australia is that melanoma is beyond the scope of superficial X-ray therapy treatment [6] although this view is not universally held and lentigo maligna is treated successfully with superficial radiotherapy in many parts of the world. (The treatment of lentigo maligna and melanoma will not be discussed further in this chapter.)
6.5 Selecting Radiation Quality
In selecting radiation quality, there must be adequate hardness of the radiation beam to penetrate to kill the deep aspect of the tumour whilst minimising harm to surrounding normal tissues.
The ultimate dose will be determined by the size, depth and anatomical location of the tumour. Thick tumours may be surgically debulked to allow them to be treated by less penetrating qualities of superficial radiation.
There are differing philosophies used at different centres to determine the X-ray quality.
6.5.1 D1/2 Philosophy
Select the radiation quality which will deliver the half-value depth (the distance from the skin surface where the skin surface dose has been reduced to 50 %) at the base of the tumour. The depth of the tumour can be estimated clinically or by histology. This approach generally utilises X-ray qualities less than HVL 1 mm Al. There is a risk of underdosing the deep aspect of the tumour if the depth is underestimated.
6.5.2 Ninety Percent Isodose Philosophy
The entire tumour should be within the 90 % isodose line to ensure homogeneity of dose. This generally utilises more penetrating radiation (or electrons). This approach is often employed by radiation oncologists who have access to more penetrating X-ray equipment and electron beam therapy and decreasing access to dermatological superficial X-ray machines.
6.5.3 An Intermediate Position
Select radiation quality which allows 70–80 % superficial dose at base of tumour. The Australasian College of Dermatologists suggests a modification of this approach where an additional margin of 2.5 mm is added to the estimated thickness of the tumour [6].
The latter two philosophies risk exposing tissues deep to the tumour to radiation [2].
In general, radiation qualities ranging from HVL 0.5 mm Al to HVL 4 mm Al will be adequate to treat skin malignancies likely to be treated by dermatological radiotherapy.
In one of the author’s practice, the approach is fairly simple: HVL 1 mm Al for thin Bowen’s disease and superficial BCCs and HVL 2 mm Al for SCCs, nodular BCCs and morpheic BCCs.
Despite these varying philosophies, it appears that actual cure rates are comparable:
Silverman et al. published results of treatment of 5,755 basal cell carcinomas treated at the New York University Skin Cancer Unit between 1,955 and 1982 – the 5-year recurrence rates were 13.2 % for curettage and electrodesiccation, 4.8 % for excision and 7.4 % for X-ray therapy [15]. And a further article on X-ray therapy (1,288 cancers) showed essentially no difference in failure rates for recurrent carcinomas versus primary tumours [16].
Caccialanza et al. reported results of 1,188 patients with 2002 primary malignant epithelial neoplasms treated from 1982 to 1995 – complete remission in 98.7 % and 5-year cure rates of 90.73 % [3].
Caccialanza et al. have also reported on recurrent basal and squamous cell carcinomas 45–70 Gy with 5-year cure rate 83.62 %, with acceptable or good cosmesis in 92.62 % of treated lesions in complete remission [4].
There are varying opinions about the suitability of morpheic basal cell carcinomas for radiotherapy –recurrence rates are higher. Bart et al. showed that morpheic basal cell carcinomas are radioresponsive [1], Wilder et al. showed that morphea-form basal cell carcinomas treated with a 1 cm margin were controlled with radiation therapy with no statistically significant difference versus other histological subtypes (using orthovoltage and electrons) [19], but Pannizon showed 22 % recurrence rate in 36 basal cell carcinomas with sclerosing component [8] utilising D1/2 approach.
It is the authors’ opinion that although Moh’s micrographic surgery or wide excision is preferable, radiotherapy can be used if larger margins both laterally and deeply are considered (1 cm margin, higher HVL).
6.6 Radiation Dose
6.6.1 Non-melanoma Skin Cancer
The biological effect of radiation is not only dependent on the dose but the time that the dose is delivered over. Neoplasms tend to repair the damage from X-rays slower and less completely than normal tissues, and therefore, if the dose is fractionated sufficiently, then the tumour will have time to accumulate lethal damage whilst allowing normal tissues to recover. In general the more fractions, the less dose per fraction is required, the longer the total treatment time and the more radiation delivered.
Although there are numerous treatment schedules practiced around the world, cure rates are roughly the same [9]. The more fractions given, the better the cosmetic result will be and the less necrosis seen [9]. It also appears that the less dose underlying tissues get, then the better the cosmetic result is likely to be. The cosmetic benefits of more fractions has to be weighed up against the often considerable logistic problems in getting elderly patients to the treatment centre. A 2–3-week course of therapy is probably just as effective as a prolonged course of treatment for small tumours [9].
Single-fraction radiotherapy for small superficial carcinoma of the skin was reported by Chan et al. [5]. For field size less than 3 cm in diameter, 20 Gy HVL 0.45–1 mm Al had less late skin necrosis compared to 22.5 Gy with no significant difference in tumour control, apart from inner canthus lesions. Although follow-up was limited to 18 months, using Kaplan-Meier analysis, the disease-free survival rate and necrosis rate was reported as 90 and 84 %, respectively, at 5 years. In another study by Hliniak et al., 20 of 25 lesions were controlled (3 years or more follow-up) with single-dose radiation (130 kV, HVL 2 mm Al 22–26 Gy). In this study for the 20 tumours in 8–16 square cm field size, the dose for 50 % tumour control was 22 Gy; 50 % necrosis dose was 24.6 Gy [12]. Trott et al. has stated that for small skin cancers less than 1 cm in diameter, single-dose irradiation is as good as any fractionation schedule – 50 % control dose was 18.2 Gy; 10 % necrosis, 20.2 Gy; and 50 % necrosis, 24.5 Gy, but as tumour size is the most important single factor determining cure that for big tumours uncomplicated 80–90 % 3-year local control can only be obtained with high total doses given in a fractionated course with low doses per fraction [17].
It is the author’s opinion that single-dose therapy should be reserved for the very infirmed or elderly patient not able to tolerate or consent to definitive surgery with small tumours who cannot attend for fractionated treatment. If possible, tumours should be debulked – one of the authors uses 20 Gy.
The factors that affect the response of tumours to fractionated therapy are multiple, and clinical data relating dose and fractionation to tumour cure and early skin side effects have been compiled into time-dose fractionation (TDF) tables – cure of non-melanoma skin cancers requiring TDF number between 90 and 110 [8, 14]. However, large or recurrent tumours probably do require higher TDF [8]. The volume of tissue irradiated also determines cure rate and side effects with larger volumes generally resulting in lower cure rates [17] and more side effects than smaller volumes unless more fractions (and hence higher total doses) are used. The overall treatment time may also affect cure – in a study by Hlinak [12] in squamous cell carcinomas treated with 60 Gy in 40 fractions, the recurrence rate decreased from 88 to 37 % as overall treatment time decreased from 70 to 45 days (only 2 of 78 tumours were less than 10 cm2 field size). Modifications to TDF and linear quadratic equation modelling have been proposed that account for the volume of tissue irradiated [13].
A number of standardised treatment schedules have been developed (see Table 6.1, [9]). The authors use a schedule of 9 × 4.5 Gy (three times per week for 3 weeks) or 6 × 6 Gy (two times per week for 3 weeks). If the tumour is larger, 15 × 3 Gy (five times per week for 3 weeks) or 30 × 2 Gy (five times per week for 6 weeks) are occasionally used.
Table 6.1
Radiation dose schedules for cutaneous neoplasms
Tumour diameter/type | Dose per fraction (cGy) | No. of fractions | Fractions per week | No. of weeks | Total dose (cGy) | TDF factor |
|---|---|---|---|---|---|---|
<4 cm | 500 | 8 | 5 | 1 3/5 | 4,000 | 108 |
500 | 10 | 5 | 2 | 5,000 | 135 | |
400 | 12 | 5 | 2 2/5 | 4,800 | 115 | |
>4 cm | 300 | 15 | 5 | 3 | 4,500 | 92 |
300 | 20 | 5 | 4 | 6,000 | 123 | |
300 | 19 | 3 | 6 | 5,400 | 102 | |
300 | 20 | 3 | 6 2/5 | 6,000 | 111 | |
BCC | 680 | 5 | 2 | 2 1/2 | 3,400 | 94 |
SCC | 680 | 8 | 2 | 4 | 5,440 | 152 |
<2 cm | 800 | 5 | 1 | 5 | 4,000 | 109 |
2–8 cm | 400 | 12 | 2 | 6 |