Stage
Description
5-year survival (%)
T
N
M
I
A
Clinical T1a
N0
M0
~100
B
Clinical T1b or T2a
M0
95
II
A
Clinical T2b or T3a
N0
M0
85
B
Clinical T3b or T4a
N0
M0
77
C
Clinical T4b N0 M0
N0
M0
65
III
A
Any clinical T
N1-3 N1a or N2a
M0
78
Pathological T1-4a
B
Pathological T1-4a
N1b, N2b or N2c
M0
62
Pathological T1-4b
N1a, N2a, or N2c
C
Pathological T1-4b
N1b or N2b
M0
50
Any T
N3
IV
Any T
Any N
M0
12
All stages
88
A genetic predisposition, particularly a Nordic Caucasian family trait with a white skin, fair or red hair and dimples, is the most important risk factor, a risk which is exaggerated by exuberant UV exposure, i.e. sunbathing and sun bench (Fig. 13.1).
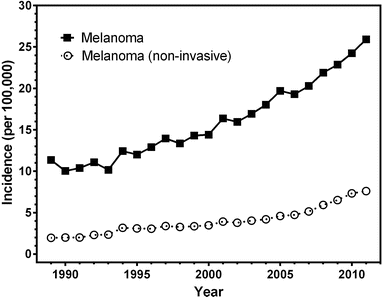

Fig. 13.1
Incidence of invasive and non-invasive melanoma in the Netherlands from 1989 until 2011 (ESR European Standardized Rate per 100,000 persons per year, adjusted for age and sex) (Source: Netherlands Cancer Registry [1])
13.1.2 Staging and Prognosis
13.2 Clinical Diagnosis
The diagnosis of a melanoma starts with a critical inspection of the patient. A popular mnemonic to remember signs and symptoms of melanoma is ‘ABCDE’ [3, 4]:
Asymmetrical skin lesion.
Border of the lesion is irregular.
Colour: melanomas usually have multiple colours.
Diameter: moles greater than 6 mm are more likely to be melanomas than smaller moles.
Evolving, i.e. changing in shape, size or aspect.
Particularly for the identification of the aggressive nodular melanoma, the ‘EFG’ acronym may better apply [5]:
Elevated
Firm to touch
Growing progressively for more than a month
13.3 Surgery
13.3.1 Surgery (Diagnosis)
Radical surgical excision is the cornerstone of both diagnosis and treatment. For a diagnostic excision, usually a 2 mm tumour-free margin is recommended. A wider excision is not advised for primary diagnosis, since in more than one third of cases, the histological diagnosis is not a melanoma. Furthermore, the prognosis is less dependent of radial extension and more from tumour depth, expressed by the Breslow thickness, which is difficult to assess by clinical observation.
If satellite or in transit metastases are present, a biopsy of one of these lesions should be taken for histological verification. If distant metastases are suspected, the diagnosis should be made by the most simple method, which usually is an incision biopsy or a fine-needle aspiration.
13.3.2 Re-excision
For a therapeutic (re-)excision, the following margins are recommended: 0.5 cm for in situ melanoma (pTis), 1 cm if the Breslow thickness ≤2 mm (pT1 and pT2) and 2 cm for Breslow thickness >2 mm (pT3 and pT4).
13.3.3 Sentinel Node Procedure and Lymph Node Dissection
The value of the sentinel node procedure in melanoma is not yet established and is not recommended beyond a clinical study.
Dissection of regional lymph node is not recommended as an elective procedure, but is indicated if regional lymph nodes are involved, i.e. inguinal, axillary or neck nodes. There is debate if such dissections should be radical or can be limited to superficial dissection or involved palpable nodes only.
13.4 Radiotherapy
The radiosensitivity of melanomas is heterogeneous, and the variation in radiation response amongst melanomas is almost as large as that reported for other human cancers differing in histological type [6]. However, based on very few clinical studies, it is mistakenly held that melanoma always is a radioresistant tumour and that the sensitivity is not much different from that of the normal skin [7, 8]. This suggests that there would only be a marginal advantage of fractionated irradiation, and the authors therefore recommended to use hypofractionated radiotherapy, i.e. the use of few but high fractions.
However, the assumption that there is only a minor fractionation effect of conventional schedules using 2.0–3.0 Gy per fraction was based on very few direct observations and was predominantly based on extrapolation of radiobiological modelling from melanoma patients that received high fraction doses (≥5 Gy) anyway and, moreover, an insufficient total doses (mostly ≤50 Gy). Further clinical studies that addressed the question of optimal fractionation, including a prospective clinical study, did not provide convincing evidence that hypofractionation is superior to conventional dose fractionation [9–14].
In hindsight, the shallow dose-response effect of melanoma radiotherapy may be biased on one hand by the fact that radiotherapy is usually reserved as palliation for patients with inoperable bulky tumours, with widespread metastases, and on the other hand by the large field sizes and inconvenient tumour sites frequently precluding the delivery of a biologically adequate dose (≥60 Gy).
13.4.1 Primary Curative Radiotherapy
Curative radiotherapy is an alternative for patients with a primary melanoma or lentigo maligna (M. Dubreuilh) unfit for surgery. This may also include patients with nodal metastasis. Many radiation schedules are being used, such as [9, 11, 14–16]:
Conventional 2 Gy per day fractionation schedule of ≥60 Gy in microscopic disease and ≥70 Gy in macroscopic disease
45 Gy in 9 fractions of 5 Gy, 2 fractions per week
36 Gy in 6 fractions of 6 Gy, 2 fractions per week
13.4.2 Adjuvant Radiotherapy
Although the role of adjuvant radiotherapy, especially after dissection of nodal metastases, is debated, recent studies suggest an improvement of loco-regional control from ~60 % after surgery alone to ~80 % after surgery plus adjuvant radiotherapy, provided that a sufficient dose is being given (≥60 Gy, conventionally fractionated) [14, 17]. However, radiotherapy does not seem to improve survival in patients with N+ disease, since distant metastasis is the major cause of tumour relapse and death in this stage [18].
13.4.3 Recurrent Melanoma
Recurrent melanoma in the scar, or in previously resected in transit metastases or lymph node stations are dreaded for pain and the risk of ulceration. Usually, a second resection is technically not feasible. As argued above, tumour localisation, metastatic spread and a poor patient condition usually preclude high-dose curative radiotherapy. Palliative radiotherapy alone therefore results in few enduring complete remissions, but a partial response resulting in worthwhile palliation of pain, ulceration or obstruction is very common [19, 20].
Radiotherapy with hyperthermia improves local control. In the four-armed randomised trial by Overgaard et al. [21], patients received either 3 × 8 Gy or 3 × 9 Gy, 1 fraction per week, with or without hyperthermia [21




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








