Medical (nonoperative)
Surgical
Dermatological
Parastomal hernia
Leakage
Poor location
Diversion colitis
Detachment
Ischemia
Retraction
Metabolic
Obstruction
Dehydration
Poor quality of life
Nephrolithiasis
Necrosis
Cholelithiasis
Prolapse
Stenosis
Infection/abscess
Disease recurrence
Oncological
Hemmorhage/varices
Complication | Incidence (%) | Average (%) |
|---|---|---|
Dermatological | 10–70 | 40 |
Parastomal hernia | 4–48 | 26 |
Poor location | 8–44 | 26 |
Disuse colitis | 0–50 | 25 |
Separation | 25 | 25 |
Retraction | 1–40 | 21 |
Obstruction | 12 | 12 |
Poor quality of life | 8–11 | 10 |
Necrosis | 1–17 | 9 |
Prolapse | 2–22 | 8 |
Infection | 1–15 | 8 |
Stenosis | 0–15 | 8 |
Ostomy Necrosis
Ostomy necrosis is more prevalent among obese patients after emergent stoma placement and in the presence of colitis or Crohn’s disease. Local trauma, a tight fascial opening, or limb devascularization can lead to necrosis via both arterial insufficiency and venous congestion [10, 27]. Necrosis is usually evident within 24 h and can be evaluated with an illuminated test tube inserted into the stoma or via endoscopy [25, 28]. Superficial discoloration with minimal necrosis rarely progresses to full-thickness necrosis and is managed by observation alone. Long-term surveillance is needed for the increased likelihood of the later development of symptomatic stenosis or retraction. Any evidence of subfascial necrosis necessitates complete revision, with special attention given to alleviating any of the possible contributing factors noted above. Laparotomy usually is required, with resection of any compromised bowel and further mobilization to ensure a tension-free stoma [25].
Ostomy Retraction
Ostomy retraction, defined as the luminal apex of the ostomy receding below the skin level, can arise from tension during construction, resolved necrosis, scar formation, as well as increased abdominal girth from obesity or loose skin from weight loss [29–31]. The principal consequence of significant retraction is an inability to properly pouch the ostomy, which can result in skin irritation, leakage, and odor. The first-line management is nonoperative with the trained tailoring of ostomy devices, preferably by an experienced enterostomal therapist [32]. For persistent complications, formal revision or relocation may be necessary, focusing on adequate mobilization of the bowel limb and ensuring at least a 10-mm protrusion for optimal pouching [10]. Abdominoplasty can be used to avoid formal revision or relocation or as an adjunct to these additional procedures. In this respect, options include careful, limited liposuction; local, open peristomal fat excision; panniculectomy; or a periostomal advancement flap [29, 33]. Patients with reasonable abdominal skin tone are more amenable to management by local measures; otherwise, more aggressive abdominoplasty may be considered [34]. Figure 43.1a–dshows potential options in the event of significant stomal retraction that does not respond to stomatherapy or eversion appliances. The alternatives can include abdominal hitching (Fig. 43.1a), peristomal fat excision (Fig. 43.1b), panniculectomy (Fig. 43.1c), and a periostomal advancement flap (Fig. 43.1d).
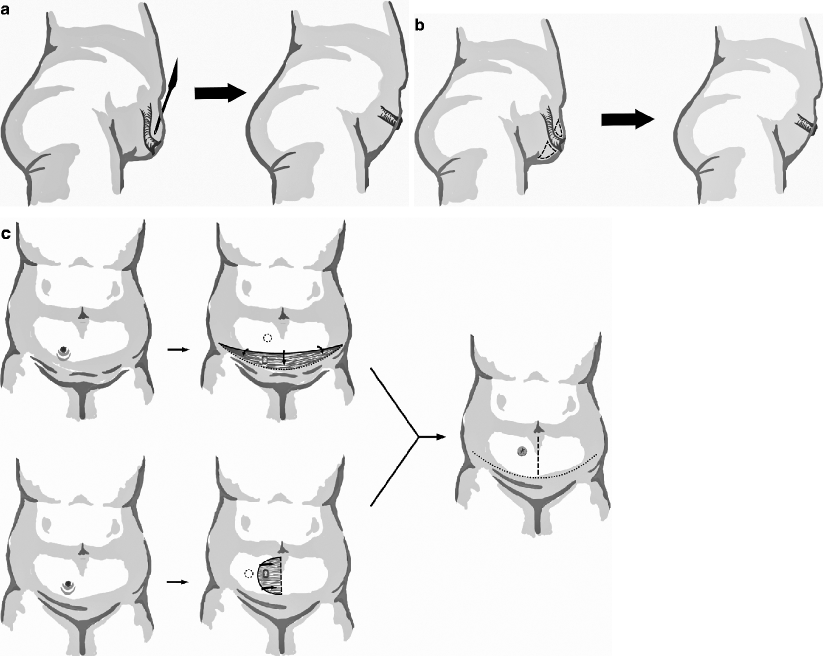

Fig. 43.1
Abdominoplasty options for ostomy retraction. (a) Liposuction. (b) Peristomal fat excision. (c) Panniculectomy. (d) Periostomal advancement flap. Note the reduced abdominal laxity and appropriate protuberant nature of the ostomy after revision, facilitating proper pouching (Illustrations by J. Jordan)
Ostomy Detachment
Similar to that mentioned above, ostomy detachment results from tension or necrosis leading to partial or circumferential mucocutaneous separation. Any deterrent of wound healing during the perioperative period, including diabetes, steroid use, or infection, may also contribute. Partial detachment generally is treated conservatively with the optimization of wound healing conditions both systemically and locally, allowing for closure by secondary intention [10]. The development of stenosis as a late complication in this scenario is frequent [28]. Complete detachment, though rare, can lead to fulminant peritonitis and therefore necessitates emergent refashioning of the ostomy [10, 27].
Ostomy Stenosis and Obstruction
Ostomy stenosis results from poor perfusion and resultant retraction and scar formation [35]. Nonoperative management can be attempted initially by avoiding foods that are poorly digested and potentially obstructing. Local dilatation generally is not effective over the long term and may actually increase scarring and worsen the stenosis. Complete revision may be required for persistent symptoms, at which time particular attention must be paid to the size of the fascial aperture [32]. A Z-plasty or W-plasty technique may be employed as an adjunct during revision to enlarge and mature the skin opening [33, 36]. Complete ostomy obstruction is differentiated by its etiology, resulting from adhesions, impaction, an incarcerated parastomal hernia, or disease recurrence such as Crohn’s disease and cancer [28]. Initial treatment is nonoperative and mirrors that of the nonostomate, with foregut decompression and fluid resuscitation. Laparotomy is required for refractory obstruction or incarceration or when there is evidence of ischemia or perforation [37].
Ostomy Prolapse
Ostomy prolapse is defined as stomal eversion through the abdominal wall (Fig. 43.2). Prolapse is more frequent with a loop as opposed to an end ostomy design; the highest incidence has been reported after construction of a transverse loop colostomy [28, 38–41]. Risk factors for ostomy prolapse include emergent construction, advanced age, poor fascial structure or a large defect, and chronic obstructive pulmonary disease [42]. Although incarceration is exceedingly rare, gentle reduction should be attempted with acute prolapse to avoid or at least delay surgery to allow for medical optimization. Reduction can be facilitated by the application of cold packs or a desiccant, such as sugar or witch hazel, to help reduce edema [43]. Subcutaneous prolapse is the protrusion of the stoma limb into the subcutaneous periostomal space (Fig. 43.2b). Although visual presentation is similar to that of a parastomal hernia (Fig. 43.2c), symptoms are local, with pouching difficulties, discomfort, and obstructive symptoms. Diagnosis is made by digital examination, noting an expansive suprafascial space, a finding that is confirmed by contrast-enhanced computed tomography scanning [37]. Subcutaneous prolapse is not amenable to the same local treatment measures as complete prolapse, but the operative management is identical. Reversal of the stoma (when tenable) is the definitive treatment for either form of ostomy prolapse. If this option is not available, other alternatives include stoma revision or formal re-siting, during which the redundant bowel is resected and the ostomy is replaced, thus ensuring a sufficiently tight fascial opening. When possible, conversion of a loop ostomy to an end ostomy results in a marginal advantage in terms of improved recurrence rates. This can be performed during revision as long as any distal obstruction is adequately addressed. A similar principle can be applied for the conversion of a complicated colostomy to an ileostomy. Unfortunately, all measures short of reversal have generally high recurrence rates [28]. Local stapled resection of the redundant bowel is a relatively novel option that can be employed to avoid general anesthesia and peritoneal exploration. The long-term outcomes of these methods, however, are presently unknown [44–49].
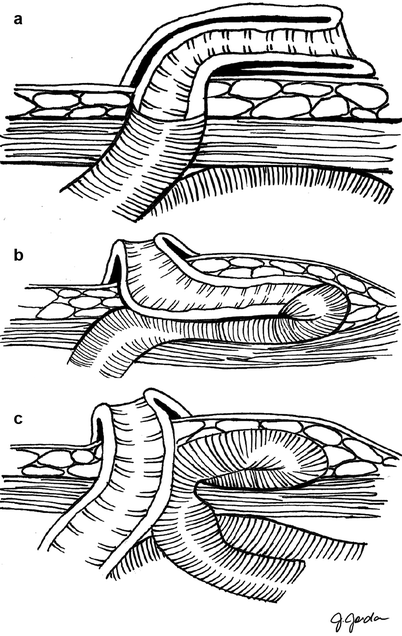

Fig. 43.2
(a) Ostomy prolapse. (b) Subcutaneous prolapse. (c), Parastomal hernia. Note the fascial defects of prolapse and subcutaneous prolapse, allowing for similar repair techniques (Illustrations by J. Jordan. Adapted with permission from Hyman and Nelson [26])
Dermatologic and Oncologic Complications
Ostomy placement results in many dermatologic complications, such as chemical and mechanical excoriation, allergic reactions, and local or systemic infection [50]. Complications are associated with persistent ostomy leakage, the type of ostomy (most notably ileostomy), and obesity [51]. Although potentially distressing, painful, and difficult to manage, intervention beyond the expertise of an enterostomal therapist is rarely required. Revision or relocation can, however, be considered for cases refractory to intensive local wound care. The primary technical detail that may contribute to a successful outcome includes the ostomy height –a flat ostomy is extremely difficult to pouch correctly. This leads to persistent peristomal irritation and excoriation, as well as the need for repeated device exchanges that further irritates the peristomal skin. Any operative intervention should pay special attention to the creation of an adequately protuberant ostomy, preferably at least 10 mm above the skin, if possible [10].
Peristomal pyoderma gangrenosum is a rare but difficult condition to manage, primarily because of irritation of the lesion during regular pouching. Definitive treatment incorporates ostomy reversal (when possible); otherwise, therapy includes the use of topical or systemic immunomodulation. Revision or re-siting of the stoma is avoided, if possible, because a recurrence rate between 40 and 100 % has been reported [32, 52].
The risk factors for peristomal infection include systemic disease (diabetes), a poor general physical condition, and immunomodulation (steroids, chemotherapy) [53]. Staphylococcusspp. and Candida albicansare the most common bacterial and fungal species isolated, respectively. Management includes proper antimicrobial selection and aggressive nutritional support as well as evaluation for local therapy or debridement of any devitalized tissue. Abscess formation requires drainage. Necrotizing fasciitis, of particular concern in immunocompromised patients, must be treated aggressively with radical resection and stomal relocation [28].
Peristomal fistulae are by far more commonly found with ileostomy; the lowest incidence occurs with loop transverse colostomies. Risk factors include repeated trauma (usually with pouching), unrecognized Crohn’s disease, and misplaced sutures that incorporate the mucosa during creation of an ostomy [27, 42]. Treatment includes optimal management of any contributing medical condition, reserving fistulotomy for persistent lesions [28]. In selected cases, the temporary deployment of a self-expanding stent may successfully manage colocutaneous fistulae without the need for repeat laparotomy [54]. Severe cutaneous Crohn’s disease is primarily managed nonoperatively with immune modulators; however, persistent ulceration or multiple fistulae may require revision or re-siting in addition to intensive medical therapy [32, 55].
Epidermal mucosal implantation results from incorporation of the bowel mucosa during ostomy creation, with consequent seeding of bowel mucosa at the mucosal-epidermal junction. This problem is preventable by primary mucodermal (as opposed to mucocutaneous) maturation of the stoma. Local measures for its treatment, such as the application of silver nitrate, can be attempted initially, but recurrence is frequent [56]. For severe cases, relocation or revision should be considered, with strict attention given to avoiding incorporation of the mucosal suture.
Although exceedingly rare, multiple oncological processes can develop on or near an ostomy, such as metachronous or synchronous bowel carcinoma after resection or unrelated cancers such as the rarely reported squamous cell carcinoma [57]. Treatment involves a full oncological evaluation with staging, wide resection, and re-siting of the ostomy in appropriate candidates [27].
Diversion Colitis
A syndrome that results from bowel diversion is disuse or diversion colitis, which manifests with abdominal and pelvic pain, lower gastrointestinal hemorrhage, and fetid mucoid rectal discharge. The definitive management is ostomy reversal. Nonoperative management is reserved for persistent symptoms to temporize them until reversal of the stoma or should reversal not be feasible. Options include 5-acetylsalicylic acid suppositories or colonic irrigation with solutions of 5-aminosalicylic acid, steroids, or short-chain fatty acids. Although rare, resection of the orphan limb may be indicated for persistent symptoms under optimized medical management as long as the permanence of the diverting ostomy is firmly established. Isolated proximal disease may be amenable to limited resection, but abdominoperineal resection is commonly required for extirpation of all affected bowel [28, 58, 59].
Stomal Varices
Significant portal hypertension, from any source, may precipitate varices at the mucocutaneous junction of the stoma, where the portal and venous systems meet [60]. Primary sclerosing cholangitis in the presence of ulcerative colitis is the strongest precipitating factor for stomal varices [61]. Initial local treatment options include direct pressure, gel foam application, suture ligation, and injection sclerotherapy, and each of these temporizing therapies has a high long-term variceal recurrence rate. Sclerotherapy in particular has the unique risk of mucosal ulceration and stricture formation [60]. Surgical options, including stomal revision, portocaval shunting, mucocutaneous disconnection (suture ligation of varices), and liver transplantation, are more effective but have significant operative risk in the presence of cirrhosis. Mesenteric venous embolization is effective but has a risk of bowel infarction from mesentericoportal thrombosis. Direct variceal embolization, which involves percutaneous placement of coils or glue within the offending varices, often is effective [62, 63]. Balloon occlusion may be used to facilitate targeting of the bleeding varix [64]. Portocaval shunting results in the lowest re-bleeding rate of all the above-mentioned therapies. As a result, the less invasive transjugular intrahepatic portosystemic shunt procedure, either alone or in combination with embolization, has become the preferred therapy for long-term relief of symptomatic variceal bleeding through correction of the underlying process. Should a transjugular intrahepatic portosystemic shunt be contraindicated, most notably in those with end-stage liver disease or encephalopathy, or in cases for which liver transplantation is unavailable, direct therapy may be the best treatment among the relatively poor group of therapeutic options.
Quality of Life in the Ostomate
An ostomy can never supplant normal anatomical function; therefore, every patient requiring stoma placement will have their quality of life (QoL) impacted to some degree [65, 66]. Even so, a patient’s QoL primarily is determined by their ability to cope with the changes brought on with an ostomy placement rather than as a result of any technical details [67], although it is influenced by complications. Therefore, during elective operations in which placement of an ostomy may be optional, the potential impact must be weighed against the need for fecal diversion on an individual basis. Patients are found to report higher QoL scores when permanent stomas are avoided, even in the presence of significant defecatory dysfunction, such as in the case of low anal anastomosis [68]. Preoperative informed consent should include a detailed discussion of the consequences of ostomy placement, both to the patient and their significant other (a factor discussed in Chap. 54) [69]. Should an ostomy result in significant physical or emotional distress, counseling and enterostomal therapeutic intervention is crucial. Timely discussion and evaluation of potential ostomy reversal follows, with continued supportive care and ongoing counseling and follow-up for those patients in whom an ostomy is deemed to be permanent [70, 71].
Recent prospective evaluation of the effect of temporary stoma use on the QoL of patients with rectal cancer undergoing sphincter-preserving surgery has shown little impact on global QoL scoring (such as the EORTC-C30/CR38 scores), including components of physical and social functioning; however, there is a distinct effect on body image, which results in diminution of sexual activity and embarrassment/privacy issues subscaling more specific, stoma-related QoL scoring [72]. The predictors of a more negative outcome in such patients include more urgent primary surgery, the distance of the stoma site from the umbilicus, older age of the patient, and the impact of the development of a parastomal hernia [6].
Poor Ostomy Location
A subset of patients suffers from poor ostomy location, an unfortunate and mostly avoidable complication. Poor locations include those that negatively affect function and hinder pouching, such as near a scar or an abdominal skin fold. In other cases, an otherwise normally functioning ostomy may cause the patient significant stress or dissatisfaction solely because of its location, such as when it lies near a pendulous breast or under the patient’s normal belt line [31, 73]. The occurrence of these problems can be significantly reduced with preoperative evaluation by an enterostomal therapist [9, 10]; however, a surgeon must be familiar with the tenets of optimal ostomy placement should the services of an enterostomal therapist be unavailable or when an ostomy is being placed emergently. A properly constructed stoma is critical because up to one third of temporary ostomies become permanent [74].
Optimal ostomy placement is made more challenging in the obese patient, where a thickened abdominal wall makes ostomy creation difficult in the traditional location. Tension-free placement is facilitated by a relatively more cephalad location, through the relatively thinner abdominal wall above the umbilicus [25]. This is of added benefit to the patient with a rotund abdomen who may have trouble reaching a lower stoma placed in a more traditional location. Ironically, patients who have experienced a substantial weight loss, with resultant loose skin, may have similar problems with the location of their ostomy and sometimes require intervention. Problems and complaints usually are manifested by the inability to properly pouch the ostomy. Counseling, in conjunction with intervention by an experienced enterostomal therapist, is sufficient in most cases. However, should these problems be refractory to supportive therapy, revision or re-siting of the ostomy should be considered.
Ostomy Relocation: Principles
Complications (refractory or otherwise) that are not amenable to local therapy require re-siting of the ostomy for definitive treatment should continued diversion be medically necessary. Relocation is often a daunting task secondary to the complications of a repeat laparotomy. General principles include relocation to another site, usually in a “mirror image” location on the opposite side of the abdomen. Strict adherence to standard ostomy formation techniques as described above is required to minimize the repeated occurrence of any complication. Management of the abdominal wall wound after fascial closure is discussed later in “Wound Management.”
Ostomy Closure
Patient Selection
Much effort has been directed toward the development of procedures that adequately divert the fecal stream and are amenable to later reversal. Although still subject to debate, most agree that loop ileostomy has proven to best satisfy these requirements; however, its reversal does have a low but significant morbidity rate of 17.3 %, a mortality rate of 0.4 %, and a repeat laparotomy rate for complications of 3.7 % [75]. The overall decision to proceed with any ostomy reversal depends on multiple patient-specific factors and no evidence of an infectious process such as a pelvic abscess or signs of an actively leaking anastomosis. A history of peritonitis or significant contamination that necessitated diversion (e.g., ruptured diverticulitis) often portends a complicated reversal. Conversely, the prior application of a bioabsorbable adhesive barrier has been shown to facilitate ostomy reversal [11, 12]. Age is consistently a significant risk factor for complications after closure [76]. Other risk factors include construction of an end ostomy, the presence of coincident diabetes mellitus, concurrent steroid or chemoradiotherapy, and a decreased albumin level. Colostomy reversals carry an increased overall morbidity relative to ileostomy reversals (a subject discussed in Chapter 45) [77, 78]. Preoperative counseling delineating the many associated risks of ostomy reversal is important and should be reviewed before creation of the ostomy, when feasible [75].
Timing of Reversal
Traditionally, there is usually a 6-week minimum interval for consideration of ostomy reversal, with 2–3 months being the average period. However, under optimal conditions, one may consider reversal of a diverting loop ileostomy as early as a few weeks [27, 28]. As stated earlier, the severity of the original pathological process that necessitated creation of the ostomy favors a longer interval to reversal, allowing for complete recovery of the patient and the diminution of any intra-abdominal adhesions [79, 80]. Significant postoperative complications, especially anastomotic leak, will delay reversal and will necessarily discourage further intervention in all but the most resilient case. Any significant delay allows many patients a period of time to accept life with an ostomy, or it may unfortunately permit disease progression, with decompensation or death before reversal [81]. All of these factors contribute to a 15–25 % incidence of presumed temporary ostomies never being reversed, even under optimal circumstances [74, 82]. Ostomy reversal concurrent with adjuvant chemotherapy or radiotherapy is particularly associated with increased complications and generally should be avoided [3]. A possible compromise is to perform stoma reversal between therapeutic cycles to both minimize disruption of adjuvant therapy and prevent an inordinate delay of ostomy reversal [81].
Preoperative Evaluation
Any anastomosis created during the initial operative procedure should be evaluated before reversal. A low pelvic anastomosis can be evaluated with digital rectal examination or proctoscopy to ensure proper healing and patency. Flexible endoscopy may be useful to evaluate more proximal anastomoses when there is any clinical suspicion of complications [83]. Contrast studies may be performed in lieu of endoscopy and may be useful as an adjunct when there are abnormal endoscopic findings [84–87].
Bowel preparation traditionally is recommended before closure. The spectrum of recommendations ranges from a simple clear (or elemental) diet before ileostomy takedown to a full mechanical upper and lower bowel preparation with antibiotic administration before a colostomy reversal. However, this decision must be made on an individual basis; recent reports have failed to demonstrate a benefit of mechanical and antibiotic bowel preparation in elective colorectal procedures [88–90].
Operative Approach
The approach to ostomy closure is most dependent on the configuration of the stoma. In most cases, a loop stoma can be reversed by a simple suture enterotomy closure or by local dissection and anastomosis [91]. Closure begins with the transverse circumferential excision of a limited area of skin surrounding the ostomy. A circular incision is made in preparation for secondary or tertiary closure or an elliptical incision is made if primary closure is planned [92]. Alternatively, the circular incision can be extended to an ellipse at the time of closure once it is certain that primary closure can be achieved. More recently, a triangular “gunsight” advancement flap has been used in stomal closure; this enhances the exposure of the mobilized bowel and cosmetically improves the skin closure site [93].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








