Primary tumor (T)
Tx
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Tis
Carcinoma in situ (Bowen’s disease, high-grade squamous intraepithelial lesion, anal intraepithelial neoplasia II–III)
T1
Tumor ≤2 cm in greatest dimension
T2
Tumor >2 cm but not >5 cm in greatest dimension
T3
Tumor >5 cm in greatest dimension
T4
Tumor of any size invades adjacent organ(s) (e.g., vagina, urethra, bladdera)
Regional lymph nodes (N)
Nx
Regional lymph nodes cannot be assessed
N0
No regional lymph node metastasis
N1
Metastasis in perirectal lymph node(s)
N2
Metastasis in unilateral internal iliac lymph node(s) and/or inguinal lymph node(s)
N3
Metastasis in perirectal and inguinal lymph nodes and/or bilateral internal iliac and/or inguinal lymph nodes
Distant metastases (M) b
M0
No distant metastasis
M1
Distant metastasis
Anatomic stage/prognostic groups
0
Tis
N0
M0
I
T1
N0
M0
II
T2
N0
M0
T3
N0
M0
IIIA
T1
N1
M0
T2
N1
M0
T3
N1
M0
T4
N0
M0
IIIB
T4
N1
M0
Any T
N2
M0
Any T
N3
M0
IV
Any T
Any N
M1
Table 18.2
American Joint Committee on Cancer/Union for International Cancer Control staging of perianal (anal margin) cancer
Primary tumor (T) | |||
Tx | Primary tumor cannot be assessed | ||
T0 | No evidence of primary tumor | ||
Tis | Carcinoma in situ | ||
T1 | Tumor 2 cm or less in greatest dimension | ||
T2 | Tumor greater than 2 cm but not more than 5 cm in greatest dimension | ||
T3 | Tumor more than 5 cm in greatest dimension | ||
Regional lymph nodes (N) | |||
Nx | Regional lymph nodes cannot be assessed | ||
N1 | No regional lymph node metastasis | ||
N2 | Regional lymph node metastases | ||
Distant metastases (M) | |||
MX | Metastases cannot be assessed | ||
M0 | No distant metastasis | ||
M1 | Distant metastasis | ||
Anatomic stage/prognostic groups | |||
0 | Tis | N0 | M0 |
I | T1 | N0 | M0 |
II | T2 | N0 | M0 |
T3 | N0 | M0 | |
III | T4 | N0 | M0 |
Any T | N1 | M0 | |
IV | Any T | Any N | M1 |
Local Disease Relapse
Twenty to 25 % of initially treated patients with anal cancer will suffer either residual disease or recurrence of disease. These patients can be offered only the chance of long-term survival by salvage surgery, which provides a 40–60 % 5-year survival rate compared with a 5 % 3-year survival for those in whom salvage surgery is not performed.
Predictors of Treatment Failure
In most series of CMT, tumor stage as assessed clinically (Tables 18.1 and 18.2) correlates strongly with prognosis [10–12]. Skin ulceration and nodal involvement before treatment also have been shown to be independent prognostic factors for local recurrence [7] and cancer-related survival [7, 13].
High-Risk Patients
Although the overall results of chemoradiotherapy are impressive, there are a number of patient groups that have a higher risk of having residual or recurrent local disease and who should undergo more rigorous follow-up after initial CMT. These consist of patients with:
1.
Bulky tumors (>5 cm in maximum dimension) or those that are invading adjacent organs at presentation (T4 tumors)
2.
Cancers in the presence of fistulae
3.
Immunocompromise, including HIV, especially in patients with poor response to or poor compliance with highly active antiretroviral therapy or who have received transplants
4.
Patients incapable of receiving full CMT
5.
Anal adenocarcinoma
Follow-Up After Primary CMT
Clinical
The full effect of CMT is seen 6–8 weeks after the treatment has been completed. A significant proportion of local treatment failures most commonly occur within 18 months, and the majority occur within 36 months of starting treatment [14]. Locally recurrent disease is almost universally palpable in the lumen of the canal or at the anal margin [15] and may be detected before becoming symptomatic; regular outpatient follow-up with a high index of suspicion is recommended. The follow-up protocol from the ACT II trial consisted of clinical examination every 2 months for the first year, every 3 months during the second year, and every 6 months from 36 to 60 months.
Differentiation between radiation effect and early recurrence can be difficult, especially if there is residual ulceration at the site of the original cancer after CMT (Fig. 18.1). Particular care must be taken with patients receiving nicorandil for the management of cardiac disease because it is well recognized that this drug can cause iatrogenic perineal ulceration that looks very similar to the ulceration seen in patients with active anal cancer. If recurrent disease is suspected, examination under anesthesia and appropriate biopsy is mandatory. The assessments of such biopsies should be undertaken by a specialist histopathologist working within the context of the multidisciplinary team. In women, care must be taken during any biopsy of a lesion on the anterior wall of the anal canal or lower rectum to avoid creating a fistula into the vagina.
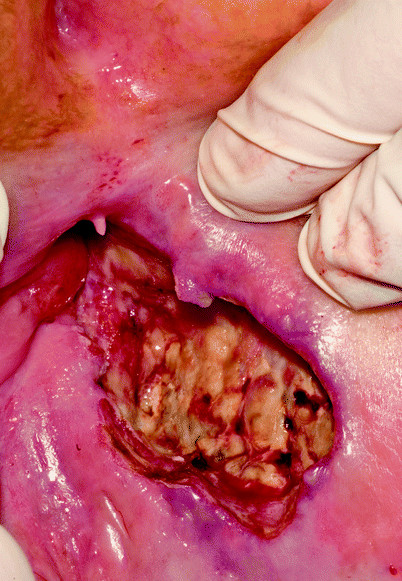

Fig. 18.1
Ulceration at site of carcinoma. Clinical distinction between radiation-induced ulceration and recurrent disease can be difficult
Radiology
Computed Tomography
Although the identification of stranding of the ischiorectal or perirectal fat associated with a mass may be predictive of tumor relapse or persistence, a study performed by Cohan et al. [16] identified a false-positive rate in 4 of 19 patients. This small study reflects the current attitude in the authors’ our unit that identification of local disease is better performed by magnetic resonance imaging (MRI). Computed tomography (CT) of the thorax, abdomen, and pelvis remains a useful modality in identifying metastatic disease that may prevent salvage surgery being performed.
Magnetic Resonance Imaging
There is little in the published literature that provides strong objective evidence of the accuracy of pelvic MRI in the identification of local disease; however, it does provide much clearer imaging of the pelvis and anal canal than CT and is part of routine practice at both initial presentations and subsequent instances where treatment failure is suspected [17].
Preoperative MRI may aid in the assessment of the degree of radial invasion beyond the anal canal, but its accuracy is reduced after chemoradiotherapy. Although findings on MRI may not alter the initial treatment (although it can), it is extremely helpful to have pretreatment images as a comparator to subsequent studies. Similarly, high signal changes on T2-weighted images of lymph nodes of the groin or pelvis may identify nodal metastases, which then should lead to targeted biopsy to establish the presence of unresectable disease. Imaging of the perineum remains problematic.
Positron Emission Tomography/CT
Positron emission tomography/CT has been shown to be a potentially accurate imaging modality in anal cancer and was capable of detecting residual or recurrent disease with a sensitivity and specificity on a per-site basis of 86–97 %, respectively, in one study [18]. It is regularly used in staging of squamous cell carcinomas of other sites and it may be of use in detecting distant metastatic disease, but its value in anal cancer is not yet proven and is not standard practice in the UK.
Serum Tumor Markers
There is no specific tumor marker that has shown any benefit in anal cancer and, certainly, there seems to be no value in measuring carcinoembryonic antigen levels in any anal malignancy with the exception of anal adenocarcinoma.
Patients should undergo protocol-driven follow-up in specialist units where there are experienced clinicians and diagnostic services familiar with the difficulties described and where the subsequent salvage treatments are available. Because of the relative rarity of this tumor and the problems it poses, there is no reason why follow-up of anal cancer should be outside high-volume specialist units that treat both primary and relapsed disease.
Salvage Surgery
Once a diagnosis of recurrence has been made, the patient should be considered for salvage surgery in the form of a radical abdominoperineal resection. Patients need to be able to withstand a major physiological challenge and metastatic disease must be excluded by CT of the thorax, abdomen, and pelvis. If there are suspicious enlarged nodes, every effort should be made to obtain tissue for histological analysis. Formal nodal biopsy rather than fine needle aspiration whenever possible is recommended. The chance of curative surgery in patients with metastatic disease in the inguinal or iliac nodes is remote, although in some rare situations it may be appropriate to perform a resection in the presence of known metastatic disease as a palliative measure.
Irradiated tissues are typically indurated and fibrotic, which makes accurate assessment of the extent of local recurrence difficult. The difficulties are demonstrated by the fact that involved resection margins may be present in approximately one third of resected anal cancer specimens – a much higher rate than for rectal cancer specimens [14]. It is essential that the dissection performed for recurrent/residual anal cancer is performed in a different manner to that done for a low rectal cancer. This can be summarized in three points:
1.
The lateral excision margins must be as wide as possible. For anal margin cancers and anal canal lesions extending to the anal margin, the skin incision should be at least 2 cm lateral to the tumor. The posterior vaginal wall should be included in the excision if involved, as it is in 70 % of all salvage procedures in women.









