Few benign melanocytic lesions encountered in clinical practice elicit the level of controversy as that generated by lesions within the spectrum of Spitz nevi. Unlike melanoma, the dermoscopic structures found in Spitz nevi tend to be distributed in a symmetric and organized manner. This review highlights the melanoma-specific structures and patterns commonly seen in Spitz nevi. Knowledge of the dermoscopic structures and patterns encountered in Spitz nevi (particularly the classic symmetric starburst pattern), together with understanding of their growth dynamics, can inform the decision whether to biopsy or monitor.
Key points
- •
Spitz nevi often display at least 1 of the dermoscopic melanoma-specific structures.
- •
The most common melanoma-specific structures seen in Spitz nevi are atypical network, negative network, crystalline structures, atypical dots and globules, streaks, blue-white veil overlying a palpable portion of the lesion, atypical blotch, and atypical vascular structures.
- •
The dermoscopic patterns most commonly associated with Spitz nevi are starburst, negative network, and thickened dark reticular, globular, and homogeneous patterns.
- •
Spitz nevi may evolve from one pattern to another during longitudinal monitoring. For example, some globular Spitz nevi may evolve into a starburst pattern and then into a homogeneous pattern before entering the phase of senescence or involution.
- •
Spitz nevi manifesting a multicomponent pattern cannot be differentiated from melanoma based solely on the clinical and dermoscopic morphology of the lesion.
Introduction
Few benign melanocytic lesions encountered in clinical practice elicit the level of angst, confusion, and controversy as that generated by lesions within the so-called spectrum of Spitz nevi. Sophie Spitz first described these benign “epithelioid and spindle cell” melanocytic neoplasms in her seminal publication in 1948. She observed that these lesions were most prevalent in childhood, and despite displaying histopathologic features that overlapped with melanoma, they usually exhibited a biological behavior commonly associated with benign neoplasms. Based on the aforementioned characteristics, these tumors were termed “benign juvenile melanomas.” Although the term “benign juvenile melanoma” is an oxymoron, it does somehow convey the conundrum faced by physicians when confronted by such lesions: Is the neoplasm truly benign? Could it represent a malignancy masquerading as a benign tumor? Is it a tumor with a higher propensity for developing melanoma? The advent of in vivo diagnostic instruments such as dermoscopy and confocal microscopy, as well as molecular diagnostic techniques have helped, to some extent, ease clinicians’ anxieties for a subset of Spitz nevi. However, for most “Spitzoid” lesions, the aforementioned questions remain unanswered and thus they are almost as disconcerting today as they were more than 6 decades ago. To complicate matters, the introduction of sentinel lymph node biopsies has even perpetuated the paradoxic notion that Spitz nevi somehow straddle 2 worlds: the benign and the malignant. This notion is based on observations that the regional sentinel lymph node in patients with Spitz nevi not uncommonly display aggregates of melanocytes with cytologic features akin to those seen in Spitz nevi. It is presently speculated that these aggregates of melanocytes in regional nodes reflect a process of passive drainage of dermal melanocytes via the lymphatics. The term “benign metastasis” sometimes used to describe this phenomenon is yet another oxymoron, and its use should be discouraged based on current understanding of biology of benign versus malignant neoplasms.
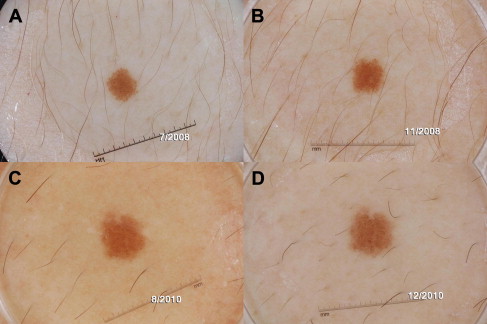
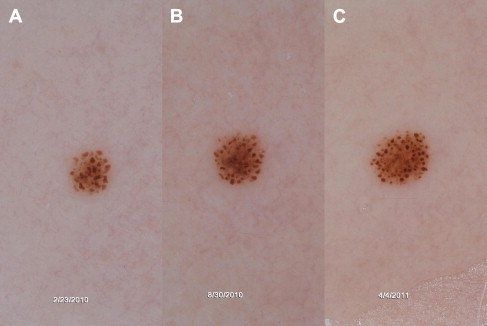
What is recurrently underscored in studies of Spitz nevi is that their clinical and histopathologic morphology often overlaps with melanoma. While Spitz nevi are benign neoplasms that do not result in widespread metastasis and death, their biology and natural history remain to be fully elucidated. Their etiology, rate of growth, and factors inducing senescence and involution remain active areas of research. Evidence is slowly accumulating that a subset of Spitz nevi, specifically those with a starburst pattern, has a predictable pattern of growth ( Figs. 1 and 2 ). In addition, it has been documented that Spitz nevi can involute. That said, it is clear that aggressive biological behavior of a subset of Spitzoid lesions, particularly those displaying rapid growth and/or lymph node involvement, does occur and can cause alarm. Based on the information currently available on Spitz nevi, management decisions often seem to be propelled by the low pretest probability of developing melanoma in children compared with adults. Thus, pediatric dermatologists are often more inclined toward conservative monitoring of Spitz nevi in children, whereas dermatologists dealing with Spitz nevi in adults often prefer to biopsy these lesions.
Introduction
Few benign melanocytic lesions encountered in clinical practice elicit the level of angst, confusion, and controversy as that generated by lesions within the so-called spectrum of Spitz nevi. Sophie Spitz first described these benign “epithelioid and spindle cell” melanocytic neoplasms in her seminal publication in 1948. She observed that these lesions were most prevalent in childhood, and despite displaying histopathologic features that overlapped with melanoma, they usually exhibited a biological behavior commonly associated with benign neoplasms. Based on the aforementioned characteristics, these tumors were termed “benign juvenile melanomas.” Although the term “benign juvenile melanoma” is an oxymoron, it does somehow convey the conundrum faced by physicians when confronted by such lesions: Is the neoplasm truly benign? Could it represent a malignancy masquerading as a benign tumor? Is it a tumor with a higher propensity for developing melanoma? The advent of in vivo diagnostic instruments such as dermoscopy and confocal microscopy, as well as molecular diagnostic techniques have helped, to some extent, ease clinicians’ anxieties for a subset of Spitz nevi. However, for most “Spitzoid” lesions, the aforementioned questions remain unanswered and thus they are almost as disconcerting today as they were more than 6 decades ago. To complicate matters, the introduction of sentinel lymph node biopsies has even perpetuated the paradoxic notion that Spitz nevi somehow straddle 2 worlds: the benign and the malignant. This notion is based on observations that the regional sentinel lymph node in patients with Spitz nevi not uncommonly display aggregates of melanocytes with cytologic features akin to those seen in Spitz nevi. It is presently speculated that these aggregates of melanocytes in regional nodes reflect a process of passive drainage of dermal melanocytes via the lymphatics. The term “benign metastasis” sometimes used to describe this phenomenon is yet another oxymoron, and its use should be discouraged based on current understanding of biology of benign versus malignant neoplasms.
What is recurrently underscored in studies of Spitz nevi is that their clinical and histopathologic morphology often overlaps with melanoma. While Spitz nevi are benign neoplasms that do not result in widespread metastasis and death, their biology and natural history remain to be fully elucidated. Their etiology, rate of growth, and factors inducing senescence and involution remain active areas of research. Evidence is slowly accumulating that a subset of Spitz nevi, specifically those with a starburst pattern, has a predictable pattern of growth ( Figs. 1 and 2 ). In addition, it has been documented that Spitz nevi can involute. That said, it is clear that aggressive biological behavior of a subset of Spitzoid lesions, particularly those displaying rapid growth and/or lymph node involvement, does occur and can cause alarm. Based on the information currently available on Spitz nevi, management decisions often seem to be propelled by the low pretest probability of developing melanoma in children compared with adults. Thus, pediatric dermatologists are often more inclined toward conservative monitoring of Spitz nevi in children, whereas dermatologists dealing with Spitz nevi in adults often prefer to biopsy these lesions.
Dermoscopic morphology of Spitz nevi
Dermoscopy-based studies have revealed a set of morphologic features that can help clinicians identify Spitz nevi. In addition, dermoscopy has been used to longitudinally monitor Spitz nevi, providing a unique opportunity to observe the biological nature of these lesions. Knowledge of the dermoscopic structures and patterns commonly encountered in Spitz nevi, together with understanding of their growth dynamics, can inform the decision whether to biopsy or monitor. Clinically Spitz nevi can be pigmented or nonpigmented, and can appear as flat or raised lesions. Dermoscopically Spitz nevi can display melanoma-specific structures; however, unlike melanoma, these structures tend to be distributed in a symmetric and organized manner within the lesion.
Dermoscopic Structures
- 1.
Atypical (irregular) network consists of a network with increased variability in the thickness and color of the lines and/or increased variability in the size of the holes of the network ( Fig. 3 ). It can be black or dark brown with a subtle blue-white veil, and it may end abruptly at the periphery of the lesion. The lines of the network correspond to melanin in the keratinocytes and/or melanocytes along the rete ridges, and the holes correspond to the suprapapillary plates. The thickened network often attests to confluent junctional nests of melanocytes. A peculiar type of network with a black reticulated appearance, described as superficial black network, has been observed in approximately 11% of biopsied Spitz nevi. Histopathologically this black network corresponds to focal areas of pigmented parakeratosis.
Fig. 3
Spitz nevus manifesting an atypical thickened network. This Spitz nevus displays a reticular pattern, characterized by an atypical, black, thickened network. The black color of the network often corresponds with the presence of melanin in the stratum corneum.
- 2.
Negative network , also known as reticular depigmentation or inverse network, consists of serpiginous hypopigmented interconnecting lines that surround elongated and irregularly shaped curvilinear brown globular structures. The exact histologic correlate of negative network remains to be elucidated, but likely corresponds to bridging of adjacent rete ridges or to large pigmented dermal nevo-melanocytic nests in the papillary dermis. Spitz nevi are more likely to harbor a negative network that is symmetrically distributed within the lesion ( Fig. 4 ), whereas melanoma is more likely to display an eccentric and/or heterogeneous negative network.





