■ The boundaries of the breast (FIG 2) are defined by the clavicle superiorly, the sternum medially, the 6th rib inferiorly, and the latissimus dorsi muscle laterally.
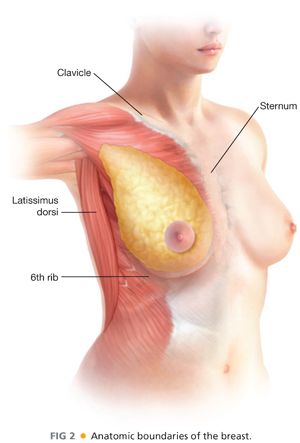
■ The axillary tail of the breast extends toward the low axilla and is called the axillary tail of Spence. Posteriorly, the breast adheres to the pectoralis major muscle and is separated by the deep fascia of the breast (see FIG 1).
■ A skin-sparing mastectomy involves preserving the skin envelope while removing the nipple–areolar complex and underlying breast via a small incision (FIG 3). Nipple-sparing mastectomy is a total skin-sparing mastectomy with preservation of the nipple–areolar complex.

■ When performing a skin-sparing mastectomy, it is important to remove as much breast tissue as possible while maintaining the viability of the skin. This is a balancing act from the oncologic standpoint to remove the majority of the breast tissue but not make the skin flaps so thin, resulting in skin necrosis and flap loss. The anatomic plane that divides the subcutaneous tissue and the underlying breast parenchyma is formed by the superficial fascia of the breast. This is an ill-defined thin layer visualized by a faint white fascial line (see FIG 1). The dissection is carefully performed in this plane. The remaining tissue on the skin envelope usually ranges between 2 and 5 mm in thickness depending on the patient’s anatomy.
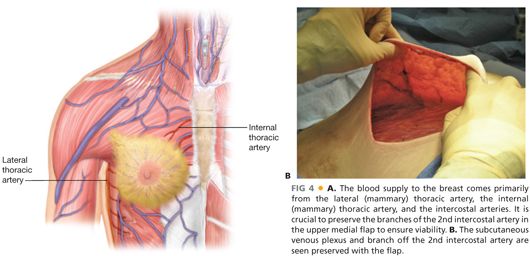
■ It is crucial to flap viability to preserve both the subcutaneous venous plexus under the skin as well as the branches of the 2nd intercostal perforator in the upper medial flap as it exits the ribs. This is the largest blood supply to the skin flap (FIG 4A,B).
■ Once the flaps are created to the clavicle superiorly including the axillary tail of Spence, medially to the sternum, laterally to the latissimus dorsi muscle, and inferiorly to the 6th rib, the breast is then removed posteriorly from the pectoralis major muscle including the underlying deep fascia overlying the muscle. It is important when creating the flap medially not to extend to the contralateral breast.
PATIENT HISTORY AND PHYSICAL FINDINGS
■ Patients with operable breast cancer are candidates for either breast conservation therapy (BCT) or skin-sparing mastectomy with or without reconstruction. It is important for patients to understand that the survival between BCT and mastectomy is equal. However, mastectomy does carry a slightly lower locoregional recurrence rate of about 2% versus 5% in 10 years after BCT.1
■ Another important difference between the two surgical treatment options is that mastectomy usually does not require postoperative radiation therapy. Postmastectomy radiation is recommended for patients with tumors greater than 5 cm in size, patients with positive margins after mastectomy, or select patients with axillary lymph node involvement for locoregional control and to improve survival.2
■ Prior to determining whether a patient will be a candidate for mastectomy or BCT, a thorough history and physical examination should be performed.
■ The medical history should focus on the patient’s family history of breast cancer in first-degree relatives and ovarian cancer in the family. Patients with a strong family history of breast or ovarian cancer, premenopausal breast cancer, patients with bilateral breast cancer, male family members with breast cancer, or those of Ashkenazi Jewish descent should be recommended for genetic counseling and testing for the BRCA 1 and 2 gene mutations. Also included in the medical history, the surgeon should ask about personal risk factors for developing breast cancer such as early menses, late menopause, a personal history of an atypical breast biopsy, lobular carcinoma in situ (LCIS), a history of previous chest wall radiation for Hodgkin’s lymphoma, or use of hormone replacement therapy. Many of these high-risk patients who decide to undergo mastectomy also opt for contralateral mastectomy at the time of mastectomy to decrease their risk of a future breast cancer. Another reason for the recent increased incidence of contralateral prophylactic mastectomy is because of patient’s anxiety, fear, and desire to reduce future risk of recurrence and cosmetic symmetry.3
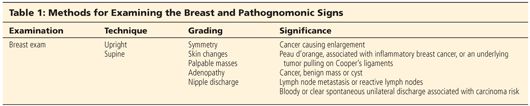
■ After obtaining a thorough medical history, a detailed physical examination would include examination of the patient in both the upright and supine positions (Table 1). While placing the patient’s hands on her hips in the upright position, the patient is visually examined for symmetry, evidence of skin changes, or dimpling. The cervical and axillary areas are palpated for adenopathy and the breasts are examined. The patient is then placed supine with both arms over her head. The breasts are examined again for palpable masses and nipple discharge, and the axillae are palpated again while lowering the arms to the side. The abdomen is then examined for hepatosplenomegaly.
IMAGING AND OTHER DIAGNOSTIC STUDIES
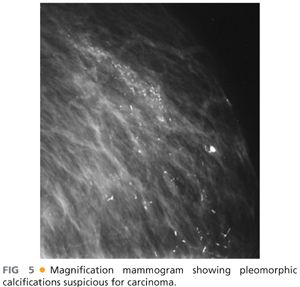
■ The purpose of preoperative imaging is to clinically stage the patient, determine operability, assist with surgical decision making, and assess the need for preoperative chemotherapy for downstaging. All patients prior to mastectomy require a recent bilateral mammogram including craniocaudal and medial lateral views. On mammogram, one should look for spiculated masses, tumor size and location, additional tumors, skin involvement, and associated calcifications (FIG 5).
■ For patients with dense breast tissue or young age, a breast sonogram may be a helpful addition to assess the presence of additional lesions in dense breast tissue and the extent and depth of the tumor.
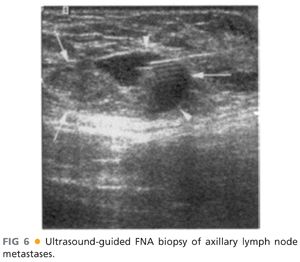
■ Ultrasonography is also helpful to assess the axilla radiographically. Suspicious nodes have a very characteristic appearance with an obliterated hilum and nondistinct borders. An ultrasound-guided fine needle aspiration (FNA) can be performed preoperatively for suspicious nodes (FIG 6).
■ Magnetic resonance imaging (MRI) of the breast is used selectively for patients who are having mastectomy. One indication is for patients with invasive lobular cancer because of a higher incidence of contralateral disease.4
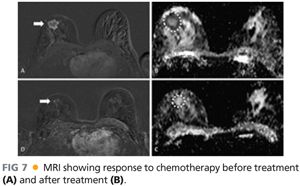
■ MRI may also be used preoperatively for high-risk patients such as those with the BRCA 1 or 2 mutation, a strong family history, dense breast tissue, difficult breast examinations, large tumors with questionable skin involvement, or those undergoing neoadjuvant chemotherapy. In patients undergoing preoperative chemotherapy, MRI helps to monitor the response to treatment. An MRI is performed before treatment begins and then repeated again after systemic treatment is completed for comparison. The MRI shows a nice relationship of the tumor location to skin distance for treatment planning and choice of surgical incision (FIG 7).
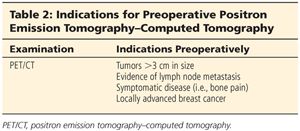
■ Staging tests to rule out metastatic disease, such as positron emission tomography–computed tomography (PET/CT) or a computed tomography (CT) scan of the chest, abdomen, and pelvis and bone scan should be considered in patients with lymph node involvement and large tumors or those patients with signs or symptoms suggestive of metastases (Table 2).
SURGICAL MANAGEMENT
■
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








