Fig. 2.1
Histology of pigmented skin, plastic embedded, semi-thick sections stained with Toluidine blue. (SB Stratum basale; SS Stratum spinosum; SG Stratum sranulosum; SC Stratum corneum)
Stratum Basale (SB)
The cells are cuboidal to columnar in shape, with a large nucleocytoplasmic ratio. The cells adhere to the basement membrane via hemidesmosomes, and with their neighboring cell with desmosomes. Tonofilaments are prominently seen in the cytoplasm, radiating from the hemidesmosomes as well as desmosomes. Other cellular organelles such as ER, mitochondria, etc., are also seen at the ultrastructural level (Fig. 2.2). In pigmented skin, many melanosomes can be seen “capping” the nucleus of the cells of SB (Fig. 2.3). Basal cells are characterized by presence of Keratins 5 and 14.
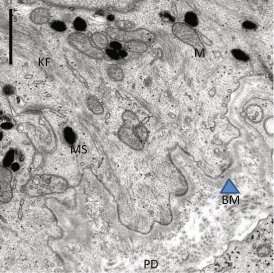
Fig. 2.2
Dermal-epidermal junction, showing papillary dermis (PA), Basement membrane (BM, Arrowhead), and basal cells (BC) shown partially. KF (Keratin filaments) and Mitochondria (M) as well as Melanosomes (MS) are seen
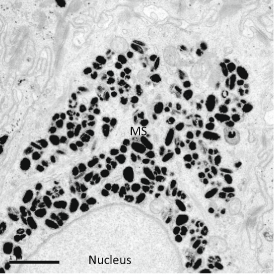
Fig. 2.3
TEM showing the microparsol of melanosomes over nucleus
Stratum Spinosum
The spinous or “prickly layer” is named so due to the large number of desmosomes that connect adjacent cells, and the prominent tonofilaments that radiate from the desmosomes. Several layers of cells are seen in this suprabasal layer. In histological preparations, large intercellular spaces (preparation artifacts) that are seen between the adjacent desmosomes gives this layer a distinct appearance. At the electron microscopic level of observation, large numbers of desmosomes mark this layer, and epidermal lamellar bodies begin to appear in the cytoplasm of cells. These organelles ranging from 0.2 to 0.5 μ size contain cholesterol, glycolipids, and fatty acids, in addition to a battery of enzymes and antimicrobial peptides. Thus the synthesis of proteins (Keratins 1 and 10) as well as lipids mark the progressive differentiation of the keratinocytes that move up being “pushed” by the newly proliferating cells below.
Stratum Granulosum (SG)
This layer is named for the presence of Keratohyalin granules that appear within the cells along with the flattening or elongation of the keratinocytes. Keratohyalin granules can be clearly identified in histological preparations (Fig. 2.1). With Electron microscopy, these granules appear irregularly shaped, and embedded within the keratin filaments. Immunoreactivity shows that they contain Profilaggrin, (whose breakdown product, Filaggrin is needed for aggregation of keratin filaments), Loricrin as well as Involucrin, two proteins that are important for the formation of the cornified envelope. Synthetic activities—both for structural proteins and barrier lipids—reach their peak at this layer. In the uppermost layer of SG, about 20 % of the cell volume is occupied by the epidermal lamellar bodies (LB), which are membrane bound structures containing stacked disc like contents. The discs can be compared to flattened liposomes, but they are actually more like pleated sheets—connected with each other (Fig. 2.4) folded like an accordion (Elias and Menon 1991). The LBs arise from the Trans-Golgi network, and remain interconnected with each other—forming a Lamellar body secretory system, positioned for secretion at a basal rate, or for a coordinated massive secretory response (Elias et al. 1998), when needed as following barrier disruption or at terminal differentiation of individual cells. As for proteins, during the process of terminal differentiation, the enzyme transglutaminase mediates the cross-linking of involucrin and loricrin, forming a thickened cornified envelope (CE) just inside of the plasma membrane. Profilaggrin is cleaved by proteases to produce filaggrin, which aggregates the keratin filaments. About the same time cellular organelles begin to get degraded through activity of proteases such as Caspases involved in the programmed cell death of keratinocytes. Thus the fully differentiated keratinocytes becomes corneocytes, the cells that make up the stratum corneum. Loss of function mutations in Filaggrins are now recognized as the basis of several skin dysfunctions ranging from sensitive skin/atopic dermatitis to several forms of Ichthyotic conditions termed disorders of cornification.(Irvine et al. 2011). In addition, tight junctions between the adjacent cells in outermost layer of SG add another barrier under the SC (Brandner 2009; Brandner and Proksch 2006)—and it is believed that they help in the polarized secretion of lamellar bodies. However, gene knock-out rodents for tight junction (TJ) proteins also fail to develop a functional permeability barrier, pointing to a highly significant role(s) of the TJs in the overall epidermal barrier formation (Proksch et al. 2008).
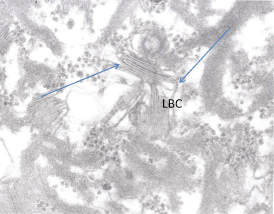
Fig. 2.4
Lamellar body contents (LBC) appear as pleated sheets (arrows) when the limiting membrane is disrupted. (Modified from Elias and Menon 1991)
Stratum Corneum (SC)
At this stage, the corneocyte is an extremely flattened (30–40 μ wide) and thin (about half a Micron in thickness) ghost of a cell filled with keratin bundles (Fig. 2.5). Further breakdown of Filaggrin leads to the production of a mixture of aminoacids collectively termed: natural moisturization factors (NMF) that allows each cell to hold moisture needed to plasticize the keratin within (Rawlings and Matts 2005). The secreted lamellar body contents, which fill the interstices of the corneocytes within the strata, undergo further catabolic modifications mediated by co-secreted enzymes, to give rise to an equimolar mixture of Cholesterol: Ceramides and fatty acids, (Fig. 2.6) which forms a lamellar structure (Wertz 2000) that occlude the intercellular space within SC—forming the permeability barrier. (Fig. 2.7). Thus, the SC is transformed to a “brick and mortar” like model—a composite material—displaying properties that are more than the sum of its components. During the terminal differentiation, desmosomes are also modified to “corneodesmosomes”: that make the corneocytes very cohesive, until the cells reach the outer most layers when programmed proteolysis of corneodesmosomes release individual cells or small units of cells that exfoliate from the surface. Such shedding of corneocytes signal the underlying cells to differentiate and join the SC-maintaining homeostasis/Autopoesis of the stratum corneum. (Hoath 2001) The number of cell layers in SC may be 10–18 cell layers varying with anatomic location. As the visible and terminally differentiated barrier layer (10 μm thick), it is often said that the SC stands between life and certain death. The characteristic, loose basket-weave appearance of this layer in histological preparations is indeed an artifact created during the dehydration and paraffin embedding protocol. Much research has been conducted on this layer, with an amazing array of techniques in the past 3 decades or so (see Elias and Feingold 2006) and what has emerged is a view that SC is not an inflexible brick wall, but an interactive boundary layer, sensing and responding to environmental changes like a “smart material” (Menon and Elias 2001). As the Stratum corneum is the perceived (oily, dry, pigmentary changes) and perceiving (touch, temperature) surface of skin it is literally and figuratively, the touch screen of the body.
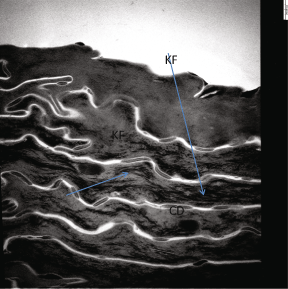
Fig. 2.5
Human stratum corneum: individual cells appear as “bags” filled with Keratin filaments (KF, arrows) and connected with other corneocytes through corneodesmosomes (CD) that appear as spot-weldings. Postfixation with OSO4, hence the intercellular lipids are not stained
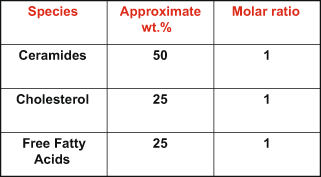
Fig. 2.6
Stratum corneum lipids: “The Big Three”
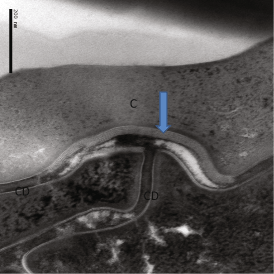
Fig. 2.7
Highly magnified view of Human SC, showing the relationship of extracellular lipid lamellae (Arrow) and corneodesmosomes (CD). Postfixation with RuO4 to reveal the lipid structures
Diverse and Interdependent Barriers in Epidermis
Although permeability barrier is the primary basis of successful terrestrial adaptation, several other environmental stressors pose challenges that need to be met by additional barriers (Menon and Kligman 2009). Skin, being the versatile organ system it is, has evolved to exploit the same structural and biochemical machinery to forge these additional barriers. An antimicrobial barrier composed of antimicrobial peptides (AMPs) such as beta defensins and cathelicidins (reviewed in Gallo and Hopper 2012) has been intensely studied in recent past. The AMPs are packaged in the same lamellar bodies that deliver the barrier lipids to the SC, closely linking the permeability and antimicrobial/innate immunity barriers. Adaptation to counter UV radiation and consequent DNA damage is reflected in the constitutive darker pigmentation in populations originally inhabiting latitudes closer to equator, where ambient UV radiation is higher. The fair-skinned races (skin types I and II) have their origin in higher latitudes where selective advantages of maximizing the low levels of UVR for Vitamin D synthesis must have been the selective force (Webb 2006; Yuen and Jablonski 2010). As the number of melanocytes are same for the dark and light skinned individuals, the activity of melanocytes and the type of melanosomes determine the level of melanin in the epidermis (Costin and Hearing 2007). The “microparsol” of melanosomes capping the nuclei of the basal keratinocytes must provide basic protection to the genomic material of these cells. Facultative pigmentation (tanning) also indicate the essential adaptive value of melanin. Additionally, efficiency of the permeability barrier repair response following experimental perturbations has been found to correlate with the degree of pigmentation of the subjects (Reed et al. 1995).
UV radiation induced oxidative damage also needs to be countered by the skin, and several biochemical (antioxidants such as Co Enzyme Q10, Vitamin E, etc.) and physiological (p53 related) mechanisms have evolved. It is worthwhile to note that the same biochemical pathway for the synthesis of cholesterol (crucial for permeability barrier) underlie the production of CoQ10 (Bentinger et al. 2010)—another example of the versatile nature of the interdependent skin barrier CoQ10 levels in the epidermis is 10 fold higher in the epidermis than in the dermis (Shindo et al. 1994). Although health implications of the use of statins, as far as CoQ10 levels have received attention (Mabuchi et al. 2005), as the epidermal cholesterol synthesis is autonomous, and as statins may not reach the epidermis (due to being metabolized in the liver), an effect on epidermal CoQ10 levels may be minimal at worst.
Immune Barriers
Sentinel cells such as the Langerhans cells (LC), positioned in the stratum granulosum, connect the epidermal barrier to the immune system. LCs internalize allergens that traverse the SC, leave the epidermis, and migrate to the lymph nodes where they sensitize the T-cells. Langerhans cells have also been reported to send their dendrites through the tight junction barriers, (Kubo et al. 2009) and in this search mode, function in “sampling” the intra epidermal environment for allergens that cross the SC barrier.
Additionally, Birbek granules of Langerhans cells contain “langerin” found to be a barrier for the transmission of HIV virus (de Witte et al. 2007).
Increasing presence of xenobiotics in our environment, due to human activity (Biocides, pharmaceuticals, industrial waste such as plastics, etc.) is a major toxicological concern. Physiological defense of skin include CYP 450 enzymes, located within the epidermal and dermal cells (Baron et al. 2001) These phase 1 enzymes catalyze introduction of functional groups into hydrophobic organic molecules. Subsequently, phase 2 enzymes help in elimination of the xenobiotics by conjugating these chemicals with hydrophilic molecules such as GSH and glucuronic acid.
While this enzyme based defense help detoxify many of the chemicals, they can also activate some of the carcinogenic compounds. Thus every cell type in the epidermis partake in the myriad defensive barrier functions of skin.
The Dermal-Epidermal Junction (DEJ) or the Basement Membrane Zone (BMZ)
The BMZ is a 0.5–1.0 μm thick band situated between the epidermis and dermis, histologically identified with periodic acid-Schiff staining. Transmission electron microscopy unraveled the complex structural components in the BMZ, (Fig. 2.2) whose major function is to anchor the epidermis to the dermis. The different regions of BMZ are (1) hemidesmosomes (that connect stratum basale with the basement membrane), (2) the lamina lucida appears electron lucent and has fine anchoring fibers connecting the hemidesmosomes. It is composed of laminins, which are heterodimers of various combinations of alpha, beta, and gamma submits, secreted by the keratinocytes. Another component is Fibronectin, which is associated with collagen fibers, and has important biological roles as well (Mosher and Furcht 1981), (3) the lamina densa (electron dense appearance), the next layer is 35–45 nm thick, and is composed mainly of type IV collagen, perlecan (heparan sulfate proteoglycan), and possibly laminin; (4) the sub-lamina densa, located below the lamina densa, the fibrillar structures connecting lamina densa to dermal plaque like structures, termed anchoring fibrils, are composed mainly of type VII collagen, secreted both by keratinocytes and fibroblasts (Marinkovich et al. 1993).
In addition to facilitating adherence of epidermis to dermis, BMZ also functions in structural support, regulation of permeability of substances from dermis to epidermis, and in embryonic differentiation. Flattening of the DEJ is a feature of aged skin, but in younger individuals, photoaged skin shows much more prominent flattening of the DEJ than sun-protected sites, and activity of Matrix Metalloproteinases (MMP) has been implicated in this alteration. Mutations that affect the components of BMZ cause heritable, blistering skin diseases.
The Dermis
Dermis, accounts for about 90 % of the weight of skin, and forms the foundation of this organ system. Two distinct zones are seen in the dermis. A Superficial papillary dermis subjacent to the BMZ appears as a loose network of connective tissue with thin collagen fibrils. Below this layer is a compact, deeper reticular layer, displaying a dense connective tissue matrix with thick and regularly oriented bundles of fibrils. The primary cell type in the dermis are fibroblasts, which produce the extracellular structural proteins, collagen, elastin as well as the glycosaminoglycans (GAGs such as Hyaluronic acid) the major water holding components of the dermis. Together, these components are known as the extracellular matrix (ECM). The early views on Dermis that is functioning merely as a structural foundation, have now been fully discarded, and the biological significance of the ECM, as well as the connective tissue fibers in skin health and disease is now fully appreciated (Gustafsson and Fassler 2000) and the physical, chemical, and biological roles of its components continue to be unraveled.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






