Rituximab is a chimeric, murine-human, monoclonal antibody against the CD20 antigen of B lymphocytes. It has been used off-label to treat and manage autoimmune and dermatologic diseases as an alternative or adjuvant therapy to systemic treatments. Due to cost, potential complications, and lack of data rituximab is used after standard systemic therapies have failed or the patient is absolutely contraindicated for corticosteroids. More research is required.
Rituximab is a chimeric murine-human monoclonal antibody against the CD20 antigen of B lymphocytes (anti-CD20 mAb). It is used in multiple medical conditions and its therapeutic role in dermatology has been increasing in the last decade. Initially used in the treatment of non-Hodgkin B-cell lymphoma, the scope of rituximab has been expanded to include autoimmune diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and chronic immune thrombocytopenic purpura syndrome. Because the B cells produce immunoglobulins, which in turn have a pathogenic role in autoimmune diseases, their depletion is surmised to result in improved symptoms and disease control. Depleting B cells inevitably decreases the activation of antigen-presenting cells and the transmission of signaling pathways to other key mediators, such as T cells.
Mechanism of action
The B-cell antigen CD20 is a transmembrane glycoprotein expressed on nearly all B cells and most B-cell lymphomas. However, it is not found on early pre-B cells or stem cells. B cells, originally arising from the bone marrow, mature by migrating through peripheral blood, lymph nodes, and spleen. In bone marrow (BM) they are known as plasma cells. CD20, specific for B cells, is expressed on B cells between the pre-B cell and pre-plasma stages. Because rituximab targets CD20, plasma cells in the BM responsible for antibody production are spared. Once rituximab binds to the CD20 protein, transmembrane signals result in altered cell-cycle differentiation and activation. Cell-mediated cytotoxicity, complement system, and direct apoptosis have been implemented in the subsequent reduction in circulating CD20+ B cells in the periphery, lymph nodes, spleen, and bone marrow.
The depletion of B-cell counts occurs within 3 days of rituximab administration and is reported to be reduced by about 90%, although variations occur. It is thought that the variations are due to the underlying disease and polymorphisms of FcγRIIIa receptors. The B-cell count remains low for 6 months and returns to baseline around 9 to 12 months after receiving 4 weekly doses of 375 mg/m 2 . In addition to depleting B cells, some data suggest that antigen-specific CD4+ T-cell numbers are reduced after the administration of rituximab, whereas no quantitative change in the different T-cell subpopulations is usually evident.
Dose and route of administration
Although rituximab is typically prescribed in weekly infusions of 375 mg/m 2 (approx. 727.5 mg per 75 kg, 1.8 m tall male) for 4 weeks, some advocate 2 intravenous infusions of 1 g, 2 weeks apart. Rituximab is also known as Rituxan or MabThera. Infusions are initially administered over 5 hours and if well tolerated, subsequent infusions can be administered over 3 to 5 hours.
Corticosteroids reduce the intensity of infusion-related adverse effects. Paracetamol and diphenhydramine are recommended before administration to decrease the likelihood and extent of infusion-related adverse events.
Monitoring of B-cell counts does not generally affect treatment because a sharp decline in absolute numbers is expected after the administration of rituximab. The aim of recurrent cycles of rituximab is to maintain circulating B cells at the lowest level for a prolonged period of time. In one report, rituximab was administered every 6 to 9 months for RA without significant adverse effects apart from reduction in serum immunoglobulin M levels. The theoretic risk of increasing the incidence of lymphomas after prolonged B-cell depletion with repeated cycles of rituximab cannot be excluded.
Routine vaccination of all patients should occur several weeks before the commencement of rituximab therapy. Patients should not receive live vaccines while receiving systemic immunosuppressive agents.
Dose and route of administration
Although rituximab is typically prescribed in weekly infusions of 375 mg/m 2 (approx. 727.5 mg per 75 kg, 1.8 m tall male) for 4 weeks, some advocate 2 intravenous infusions of 1 g, 2 weeks apart. Rituximab is also known as Rituxan or MabThera. Infusions are initially administered over 5 hours and if well tolerated, subsequent infusions can be administered over 3 to 5 hours.
Corticosteroids reduce the intensity of infusion-related adverse effects. Paracetamol and diphenhydramine are recommended before administration to decrease the likelihood and extent of infusion-related adverse events.
Monitoring of B-cell counts does not generally affect treatment because a sharp decline in absolute numbers is expected after the administration of rituximab. The aim of recurrent cycles of rituximab is to maintain circulating B cells at the lowest level for a prolonged period of time. In one report, rituximab was administered every 6 to 9 months for RA without significant adverse effects apart from reduction in serum immunoglobulin M levels. The theoretic risk of increasing the incidence of lymphomas after prolonged B-cell depletion with repeated cycles of rituximab cannot be excluded.
Routine vaccination of all patients should occur several weeks before the commencement of rituximab therapy. Patients should not receive live vaccines while receiving systemic immunosuppressive agents.
Adverse effects
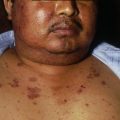
Polymorphisms of the FcγRIIIa receptor may influence the efficacy of rituximab and the extent of associated adverse effects. The most frequent adverse effects are transient, infusion-related, and mild to moderate in severity. They include fever, headache, nausea, chills, orthostatic hypotension, mucocutaneous reactions, and thrombocytopenia. Severe cytokine release syndrome can occur during the first infusion. It is of major importance not to forget infections, which can be severe. There was 1 case of septicemia leading to death in the series of 21 patients reported by Joly and colleagues, 1 of 7 patients died in the series studied by Hertl, and 2 of 17 patients died in the cicatricial pemphigoid series from St Louis, which approximately corresponds to 4 deaths among 44 patients (almost 10%). These severe infections seem favored by older age and the concomitant use of high doses of steroids and/or immunosuppressants. There have been a significant number of reports of progressive multifocal leukoencephalopathy in patients concomitantly treated with rituximab and polychemotherapy for B-cell lymphoma and some reported cases in patients with nondermatologic autoimmune diseases such as SLE, RA, and Wegener granulomatosis.
Although it has been theorized and confirmed by many reports that serum levels of immunoglobulin G (IgG) remain unchanged because stem cells and plasma cells do not contain CD20 and are not affected, few case reports have reported reduced levels of immunoglobulins. Anemia, neutropenia, and thrombocytopenia have also been reported in 1% to 7% of patients after the fourth infusion. In the context of these findings, it is important to regularly monitor blood counts in patients treated with rituximab.
Use in autoimmune blistering diseases
Autoimmune blistering diseases (AIBD) are a heterogeneous group of blistering diseases affecting the skin and/or mucous membrane. Although these diseases are typically managed with systemic corticosteroids in combination with immunosuppressants or immunomodulators, inefficacy (recalcitrant and relapsing types), contraindications, and adverse effects are reasons for choosing an alternative therapeutic option. This is especially the case with pemphigus and mucous membrane pemphigoid, which often require prolonged administration of systemic corticosteroids and other adjuvant systemic immunosuppressive agents. Rituximab has therefore been used as an alternative and/or adjuvant to other therapies.
Pemphigus
Pemphigus is a rare mucocutaneous disease characterized by antibodies to adhesion protein, desmoglein (Dsg) 1 and 3. Pemphigus vulgaris (PV) and pemphigus foliaceus (PF) are the 2 main types of pemphigus usually managed with systemic corticosteroids. Adjuvant therapies such as mycophenolate, azathioprine, methotrexate cyclophosphamide, and cyclosporine are used with varying degrees of efficacy.
The use of rituximab in pemphigus was first reported in 2002 and since then there have been many case reports/series demonstrating favorable results, especially in patients who have not responded to more standard treatments. A study assessing the resolution of pemphigus lesions with intravenous immunoglobulin (IVIG) and rituximab was published in 2006. Eleven patients with recalcitrant disease were treated with rituximab (375 mg/m 2 ) once a week for 3 weeks and with IVIG (2 g/kg) in the fourth week. This cycle was repeated for month 2. During months 3 to 6, these patients were administered one infusion of rituximab and IVIG. By the end of the second cycle, 9 of 11 patients (82%) had resolution of lesions, which was maintained for more than 20 months. The combination of IVIG with rituximab was not compared with rituximab alone, and hence it is uncertain whether IVIG adds any benefit to rituximab alone.
Two patients with recalcitrant PV were successfully treated with 2 courses of rituximab. The 2 reports on these cases implied better outcomes with 2 cycles of rituximab rather than 1 but because these are only isolated case reports, it is difficult to draw any firm conclusions. In 2007, however, a multicenter open-label French trial assessing the effect of a single cycle of rituximab alone in pemphigus was reported. Twenty-one patients (14 with PV, 7 with PF) who failed to respond, be maintained, or had a contraindication to oral corticosteroids were given a single cycle of rituximab. Each patient received 1 infusion (375 mg/m 2 ) weekly for 4 weeks, with immunologic evaluations at regular intervals. Eighteen of 21 patients (86%) had complete remission at 3 months and 2 of 21 patients (10%) had complete remission by 12 months. Of those who had complete remission, 9 (45%) patients relapsed after a mean of 19 months, 2 of whom required a second cycle of rituximab. This study also demonstrated the corticosteroid-sparing effect of rituximab, with 8 of 21 (38%) patients not requiring any systemic treatment at the end of the study. A relationship between disease activity and antidesmoglein antibody (anti-DSG) levels was evident by the reduction of anti-DSG-1 and, to a lesser degree, anti-DSG-3 titers in patients who had experienced remission at 3 months. Rituximab does not seem to influence the mean IgG levels, which did not change significantly.
In a study in 2006 from Germany, rituximab was administered to 7 patients with AIBD (4 with PV, 2 with bullous pemphigoid (BP), and 1 with mucous membrane pemphigoid [MMP]). Six of 7 patients received weekly infusions (375 mg/m 2 ) for 4 weeks. Although complete resolution or reduction in lesion size by more than 50% occurred in 6 of 7 patients (86%) at 13 months, 4 of 7 patients (57%) had severe adverse effects including blindness and death secondary to bacterial pneumonia.
Maintenance therapy at a lower dose may be an option in the treatment of pemphigus that requires further evaluation.
MMP/Epidermolysis Bullosa Acquisita
MMP refers to a heterogeneous group of AIBD typically affecting the mucous membranes. MMP is associated with significant morbidity secondary to scarring and ophthalmologic complications. Typical histologic findings include IgG and C3 depositions in the basement membrane zone. MMP, previously known as cicatricial pemphigoid, is a potentially severe AIBD with an incidence of 1 per 1,000,000 per year with a mean age of 70 years. Epidermolysis bullosa acquisita (EBA) has an incidence of 0.2 new cases per 1,000,000 inhabitants per year. The use of rituximab in MMP and EBA has been limited to a few case reports. Combination therapy with IVIG has been reported to be effective in ocular cicatricial pemphigoid. In a recent study, 25 patients with severe refractory MMP were treated with 1 to 2 cycles of rituximab (375 mg/m 2 ) for 4 weeks. Within a median of 12 weeks of having 1 cycle, 17 patients had complete resolution of their lesions. Five of the 8 remaining patients experienced complete resolution after receiving a second cycle of rituximab. Although 22 of 25 patients (88%) had complete resolution of both ocular and extraocular lesions, 10 of 22 patients (45%) had a relapse after a mean of only 4 months. Significant adverse events occurred in this study, with 3 patients experiencing infections, 2 of whom died. Despite the severity of adverse outcomes, it can be argued that in the case of MMP, it is important to quickly achieve disease stabilization and remission as disease progression often leads to blindness, laryngeal, and esophageal complications. The benefits versus the risks must always be considered before administration of rituximab.
Rituximab has been suggested as a potential corticosteroid-sparing agent, reducing cumulative doses of systemic corticosteroids, although this has not been demonstrated in a randomized controlled trial.
BP
Although BP is the most common AIBD, the use of rituximab is infrequently reported, probably because BP responds readily to potent topical corticosteroids. Typically affecting the elderly with high mortality, BP presents with multiple bullae, erosions, and severe pruritus, and only rarely has mucous membrane involvement. Two female patients with concomitant BP and chronic lymphocytic leukemia were both treated with rituximab (375 mg/m 2 weekly for 4 consecutive weeks) alone after inadequate response to corticosteroids and antihistamines. Maintenance therapy of 1 dose every 2 months was initiated and both patients had complete remission after this treatment, even at the 3-year follow-up.
Successful use of rituximab has been reported in a 5-month old infant who had unsuccessful systemic treatments including corticosteroids, dapsone, IVIG, and cyclosporine. Two doses of rituximab were added to the therapeutic regimen 4 weeks apart (375 mg/m 2 and 187.5 mg/m 2 ), and improvement was noticed within days.
A retrospective study looking at 7 patients (5 with BP and 2 with MMP) treated with rituximab found that complete and partial remission was achieved in 4 patients with BP and 2 patients with MMP. The main indications for the use of rituximab in BP patients are not clearly defined because of the extremely high efficacy of ultrapotent topical corticosteroids during the acute phase of the disease and the numerous drugs proposed for maintenance therapy (oral corticosteroids, low doses of methotrexate, tetracyclines). Potential indications for the administration of rituximab in BP might be: (1) recalcitrant types of BP (extremely rare) and (2) patients with multiple relapses, who are observed quite frequently.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree