Revision of Implant Breast Reconstruction
Breast reconstruction with implants remains very popular. It represents the majority of breast reconstructions performed in the United States.1 Since the introduction of tissue expansion for breast reconstruction,2 the most common method of implant-based breast reconstruction is a two-stage process of tissue expansion followed by the insertion of either a silicone gel or saline-filled breast implant.
The essential goals of breast reconstruction remain creating a breast mound, maximizing symmetry between the reconstructed mound and the contralateral breast, and reconstructing a nipple in the appropriate position to transform the mound into a true breast facsimile. A final finishing touch is providing skin pigmentation to the nipple and areola area to produce the best possible color patch symmetry between the reconstructed nipple and the nipple areola complex (NAC) of the opposite breast. This process occurs in stages, and in fact breast reconstruction in every patient occurs as a continuum. This must be stressed to each patient who is seen in consultation for breast reconstruction. This is especially true for implant-based breast reconstruction, which as stated is almost always a two-stage process consisting of the placement of a tissue expander followed by the placement of an implant.
Although prosthetic breast reconstruction is a simpler procedure for the patient, I find it much harder to obtain consistently good results with implant-based breast reconstructions than with autologous tissue methods. It must be borne in mind by the surgeon that by their very nature such procedures are much more likely to require surgical revision, especially with the passage of time.3 This fact must be explained to every patient preoperatively.
There are many challenging problems and situations that may present themselves in the course of implant-based breast reconstruction, either between the positioning of the tissue expander and the planned implant exchange, or after the second stage when a saline-filled or silicone gel-filled implant has been placed. This chapter will discuss my approach to treating problems encountered at either stage in this process.
Most often these problems include capsular contracture around the previously positioned implant, asymmetries relating to improper expander or implant size (volume or base width), implant or expander malposition, inframammary (IM) fold asymmetries, skin rippling with the appearance of ripples or folds, compromised local covering soft tissue, saline implant deflation, and silicone gel implant rupture.4 In addition, there may be breast asymmetries that are most often derived from a combination of all of the above, or are related to the patient’s opposite breast, which may not be inherently well matched by the placement of an implant.
This chapter focuses on the most commonly encountered problems seen in patients who are in the implant-based breast reconstruction continuum, with the exception of implant rupture, which is covered in the chapter on explantation (Chapter 4). These challenges are often complex, but if handled appropriately the outcome can be very satisfying for the patient and rewarding for the surgeon.
PREOPERATIVE EVALUATION
When evaluating a patient who has a problem during or after any of the stages of implant breast reconstruction, it is essential to obtain a careful history and to perform a systematic and compulsive physical examination. If the patient has been previously operated on by a different surgeon(s), I find it helpful to obtain the previous operative reports so that I have precise information about implant type, position, and volumes. Only with this information is it possible to accurately assess the factors that may be contributing to the specific problematic situation with a given breast reconstruction.
There are four issues for the surgeon to consider in evaluating each particular patient. First, it is important for the treating surgeon to determine whether an implant-based reconstruction is or was a reasonable or even feasible operation in the first place for a given patient. In many patients it is the “default option.” By this I mean that this method is chosen because there is no other good option. The typical scenario is a patient with significant skin deficiency (>6 cm in the vertical or horizontal dimension— a situation where the addition of flap tissue is highly preferable but declined by the patient) who is reconstructed with an implant, or a patient with extremely thin
local tissues who exhibits a poor result because of inadequate covering tissues. The surgeon must understand what local conditions may have existed that would most likely have predisposed a suboptimal outcome such as significant scars at the site of breast reconstruction, previous infection, and most importantly antecedent radiation therapy. It is also important to know if there was a history of failure of a previous breast reconstruction.
local tissues who exhibits a poor result because of inadequate covering tissues. The surgeon must understand what local conditions may have existed that would most likely have predisposed a suboptimal outcome such as significant scars at the site of breast reconstruction, previous infection, and most importantly antecedent radiation therapy. It is also important to know if there was a history of failure of a previous breast reconstruction.
A second issue relates to the implant that was used. It is important for the surgeon to decide if the appropriate implant or tissue expander was used for the initial stage(s) of the reconstruction, i.e., whether the device had sufficient surface dimensions in the form of base width, height, and volume to appropriately contour the local soft tissues to best match the opposite breast.
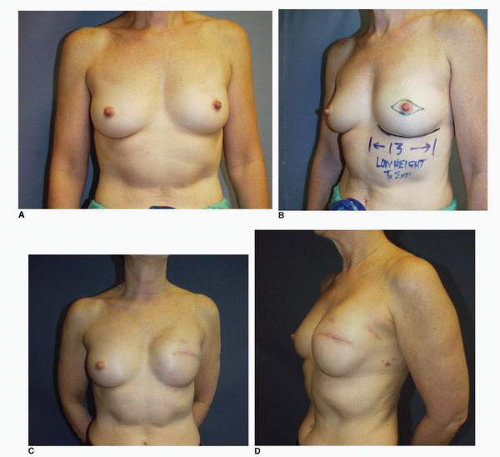
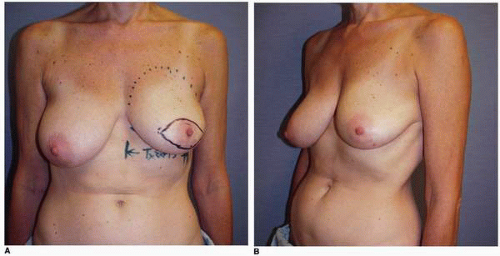
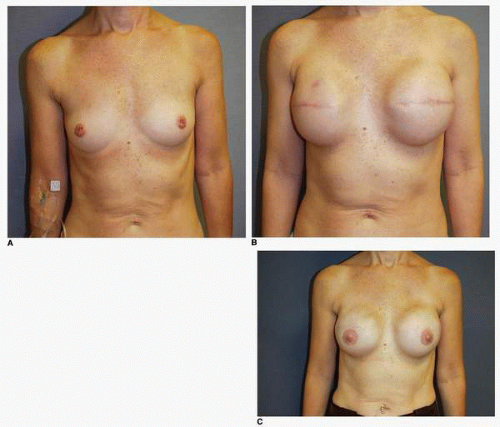
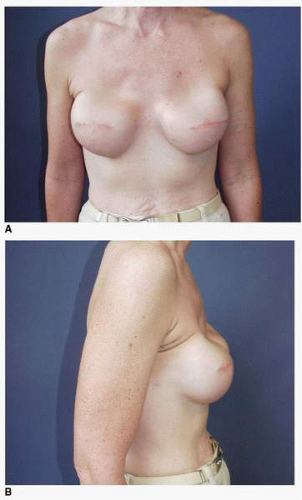
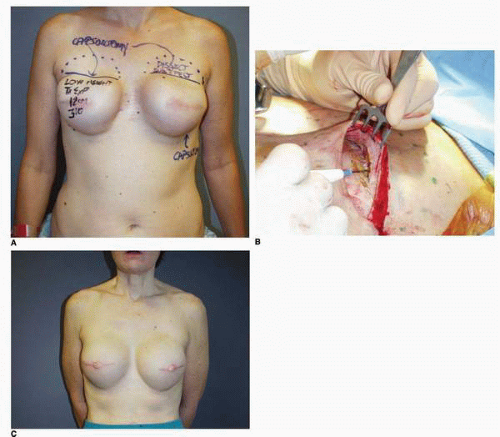
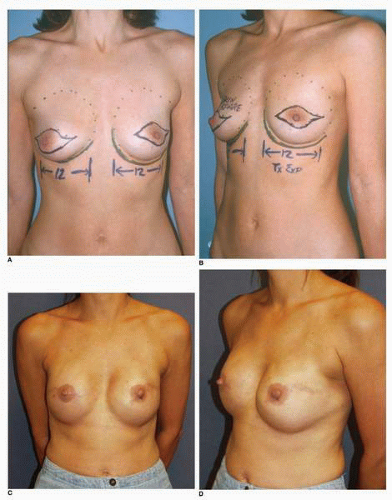
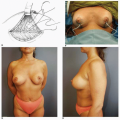
Third, if the patient’s problem is asymmetry, the surgeon must determine whether opposite breast modification in the form of breast augmentation, mastopexy, augmentation mastopexy, or reduction, all of which may sometimes be necessary to optimize symmetry, was discussed before surgery. In my experience, it is most often difficult, if not impossible, to achieve really good symmetry by placing an implant beneath tissues remaining after a mastectomy without placing an implant on the opposite side as either a breast augmentation or augmentation mastopexy. Said another way, I believe that patients in whom it is possible to achieve truly excellent symmetry with an implant on one side and no surgery on the opposite breast are indeed very rare. In these rare individuals the opposite breast almost looks like an implant, with a round appearance (Fig. 6-1).
Finally, the surgeon must ask whether there has been any intervening condition that has changed the appearance of the previous implant reconstruction. The most common condition in this context is radiation therapy. In my experience, radiation administered at any stage of the process of implant-based breast reconstruction usually produces an accelerated form of capsular contracture, which often results in a profound change in the visual and tactile characteristics of the reconstructed breast (Fig. 6-2). Such a situation is most often an indication for the addition of flap tissue to address the firmness in the existing tissues.5
I have found that in the setting of an implant reconstruction, modification of the opposite breast is a common consideration (Fig. 6-3A). This can be discussed in a simple and straightforward manner with the patient. I find that having the patient view photographs of other patients who have undergone the combination of implant reconstruction on the side of mastectomy and the placement of an implant with or without mastopexy (Fig. 6-3B) on the opposite breast is very helpful and educational for her. It is also important to show the patient photographs of outcomes in other patients who have not undergone this combination (Fig. 6-4). The patient can then decide whether to have the opposite breast modified. I have a book containing photographs of each procedure I perform available in the office for patients to review before their surgery.
The treating surgeon must explain the risks and benefits of opposite breast modification. The risks include scars on the breast, loss of nipple sensation and decreased sensation in the skin of the breast, alteration of breast parenchyma, and decreased sensitivity to mammography when an implant is placed. In the latter situation the amount of tissue that is hidden from the mammographer depends on the size of the breast, the size and position of the implant, and the presence and degree of capsular contracture.6 For this reason, in the setting of a previous contralateral breast cancer, I place virtually all implants in the submuscular position. In this location the risk of capsular contracture is reduced and the interference with mammography is minimized. I have performed opposite breast modification with increasing frequency over the past decade, to the point where a significant majority (>60%) of my patients have the opposite breast adjusted when an implant reconstruction is performed.
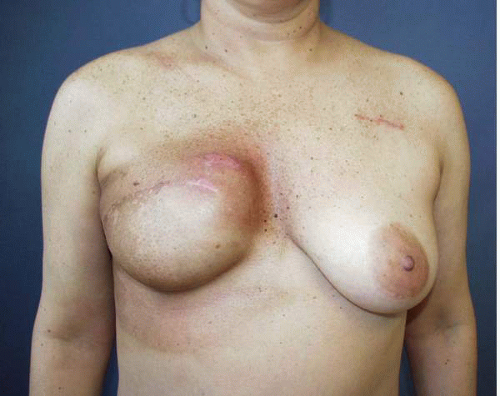 FIGURE 6-2. Appearance of right breast following completion of radiation therapy in patient with tissue expander in place. Skin changes are those of subacute and chronic radiation injury. |
As noted, options for surgery on the opposite breast include augmentation, augmentation with mastopexy, mastopexy alone, and even breast reduction. It has been my experience that a vertical mastopexy technique7 can often produce a more round and implant-like appearance in the contralateral breast (Fig. 6-3). However, the best results usually entail implant placement on the opposite side, which gives the contralateral breast a rounded look that best matches an implant reconstruction (Fig. 6-5).
SELECTING THE RIGHT DEVICE
Begin with the end in mind.
As outlined in Chapter 2 and Chapter 3, Fig. 3-1A, there are many implant options for the surgeon to choose from. Good results in a given patient can be obtained from using any number of implants. Surgeons tend to use implants (and tissue expanders) they are experienced and comfortable with from the standpoint of predictably producing good results in their hands. Different implants may be used in different situations. Variability in patient anatomy, including chest wall configuration, skin conditions, and tissue deficits, may encourage the surgeon to select a certain implant over another in a given situation.
There are very few ironclad rules or stipulations regarding the choice of an implant for breast reconstruction. As noted in Chapters 2 and 3, the implant selected should have the appropriate base width, volume, and vertical dimension to produce the desired contour and shape in the reconstructed breast. The analysis of the curves, shape, and relationship of the opposite breast to the chest wall is reviewed in Chapter 2.
Following a thorough discussion with the particular patient about her goals and a careful examination of the breasts, the surgeon must be able to envision what he or she is trying to create and then select the appropriate implant(s) for that situation. Presently in my practice implant reconstruction is almost always (>98% of the time) done in stages, with the placement of a tissue expander as the first stage, followed some time later by the placement of an implant. Therefore it is important for me to select the appropriate tissue expander, as well as the appropriate implant.
BEGIN WITH THE END IN MIND
Selection of the tissue expander for a given patient starts with selection of the implant(s). The choice of implant for breast reconstruction is largely determined by the base width of the breast, by the desired breast volume, to some degree by the vertical height of the breast, and by whether the patient chooses to have a contralateral breast augmentation. After the evaluation is complete, I find that it is helpful to refer to the implant charts compiled by each of the implant manufacturers (see Fig. 3-5) for the final implant selection. The surgeon must pick a tissue expander that will accommodate the base width of the implant that will be used. Therefore, I try to always select the implant I will use before I place the expander.
In most cases the choice comes down to either of two expanders, with one having a slightly larger base width than the other (e.g., a 12-cm versus a 13-cm expander). In most cases I choose the larger device for a variety of reasons. A larger space will allow better movement of the implant within the periprosthetic capsular space because my preference is to use a smooth-walled implant. Additionally, if the periprosthetic space is of slightly larger dimension than the selected implant, it can be reduced in volume and altered in its dimension or changed in its position (e.g., to a lower level) in a fairly precise way by a suture capsulorrhaphy or direct excision of the capsule with surgical repair. This is especially common if the patient elects to undergo contralateral breast augmentation because the larger expander will accommodate the larger implant needed on the side of the reconstruction to produce volume symmetry with the augmented opposite breast.
I have found that the best appearance of the reconstructed breast is achieved when there is little or no manipulation of the lower portion of the periprosthetic capsule because this is the area that accounts for the smooth appearance of the IM fold and lower pole of the reconstructed breast. However, in most cases the volume and internal dimensions of the periprosthetic capsular space can be increased by performing appropriately positioned capsulotomies. In this way progressively larger implants can be accommodated in a given periprosthetic capsular space that has been created by the tissue expander. This is commonly done at the time of exchange of the tissue expander for the implant that will be used for the breast reconstruction. Similarly, the volume of the periprosthetic space can be decreased by performing the necessary capsulorrhaphy(ies) before implant placement.
In summary, when performing implant-based breast reconstruction, choose the implant first. This entails selecting a device of sufficient base width, height, and volume to produce the appearance of visual symmetry with the opposite breast when viewed in the frontal position. For implant breast reconstruction (and breast augmentation to a great degree), device selection is governed by dimensional concepts8 (Chapters 2 and 3). This is especially true when using saline-filled implants. The volume contained in these breast implants can vary depending on projection or profile of the device, i.e., a high-profile implant contains a greater volume of filler for a given base dimension (diameter) of the implant.
The implant selection for a given patient is determined by the factors previously reviewed, the most important of which is the base dimension of the opposite breast. The fill volume and projection are also important. Different volumes are contained within a given base diameter of an implant depending on its projection specifications. These specifications are listed on the charts issued by the implant manufacturers (see Fig. 3-5). Selection of the tissue expander is then made to create a periprosthetic capsular space that will accommodate this implant.
PREOPERATIVE PATIENT ASSESSMENT
I now believe that for breast reconstruction, tissue expansion is more of a tissue-molding process than it is a process of tissue expansion or skin stretching per se. That is to say, I believe that the results of the tissue expansion process in many ways represent more of a loan than a dividend. For that reason, I believe that when the measured or anticipated skin tissue deficiency in a given patient is more than 4 cm, this is an indication for a flap reconstruction rather than a tissue expander insertion. That said, the majority of breast reconstructions in my practice are done in stages by first creating a periprosthetic capsular space by shaping and molding the tissues in the lower pole of the new breast, then placing the implant.
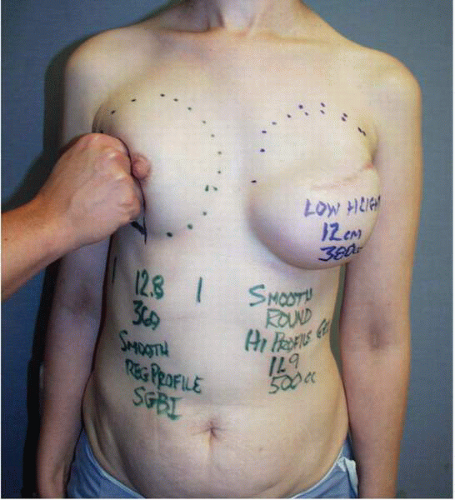
As noted several times earlier in this chapter, when tissue expansion is chosen, the surgeon must select a tissue expander with the appropriate base width for a given patient. This dimension is determined by measuring the base width of the patient’s opposite breast from the parasternal area to the anterior axillary line in the frontal plane [anteroposterior (AP) view; Fig. 6-6]. This can be done using a caliper or tape measure.
The height or superior extent of the opposite breast is also noted. It is the most important dimension for selecting the implant that will be used to complete the second stage of the reconstruction. This dimension is most accurately determined by gently displacing the opposite breast posteriorly against the chest wall with the ulnar side of the examiner’s hand (Fig. 6-7) and noting the superior extent of the breast fullness. I usually outline the dimensions with dots placed on the skin (Fig. 6-7). This vertical dimension from the dots to the lower contour of the breast is recorded in the chart for each patient because it serves as a guide for the selection of the implant.
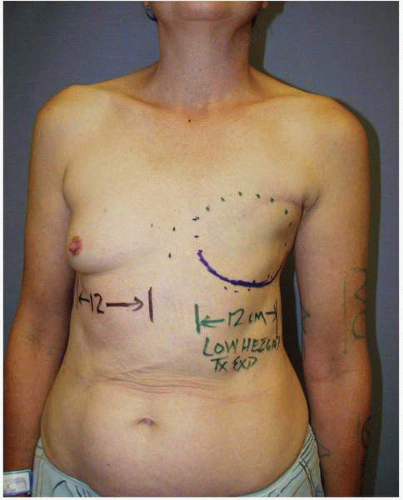 FIGURE 6-6. Measurement of base dimension of opposite breast serves as guide to selection of appropriate implant and, before that, correct tissue expander for breast reconstruction. |
This vertical dimension is not nearly as important for selecting a tissue expander as it is for selecting the implant. I now almost exclusively use a low-height tissue expander (Fig. 6-8). However, I record the breast height in the patient’s chart and refer to it before I select the implant. Suffice it to say that the key dimension in selecting a tissue expander is the base width of the contralateral breast (see Fig. 2-3). Initial tissue expander positioning is important in that a very accurate placement of a tissue expander sets the stage for the best possible result in terms of permanent implant position and breast appearance.
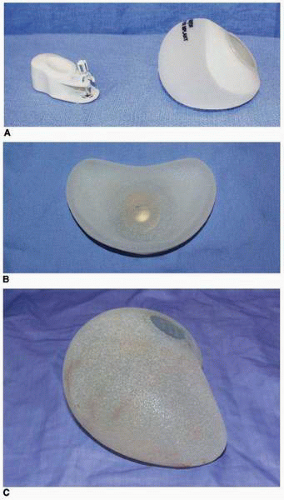
Currently available tissue expanders for breast reconstruction include single-chamber saline devices (Figs. 6-8A,B and 6-9), dual-chamber saline devices (PMT), and combination saline-silicone devices [e.g., Becker (Mentor Corp., Santa Barbara, Calif)]. For breast reconstruction procedures, virtually all expanders are textured devices. Most have integrated ports, but some, such as the Becker expander/implant and the Spectrum (Mentor Corp., Santa Barbara, Calif) (Fig. 6-10), have remote fill ports.
As stated, when planning a breast reconstruction I always “begin with the end in mind” from the standpoint of selecting an implant. In the United States both manufacturers (Mentor Corp. and INAMED Medical, Santa Barbara, Calif) offer a wide variety of implant shapes, projection profiles, and surface characteristics, along with brochures and charts I refer to constantly when dimensionally planning an implant reconstruction (see Fig. 3-5). Both silicone gel and saline-filled implants are used in my practice, but the distinct majority of my breast reconstruction patients request the silicone gel-filled breast implant. The cohesive gel implant is gaining wide popularity in Europe and South America. My early experience with that device has been a very positive one from the standpoint of breast shape.
As stated, each surgeon has his or her own preferences in terms of implants for breast reconstruction. I feel that I am currently achieving the most consistently good results with the use of a high-profile smooth-walled silicone gel implant placed after the first-stage tissue expansion is completed with a low-height tissue expander. As noted in Chapter 3, I am big believer in implant displacement exercises as a means of promoting implant (and breast) softness. Therefore I have all patients who receive smooth implants for breast reconstruction (as well as for breast augmentation) perform implant displacement exercises twice a day—forever. I believe this is helpful following the placement of a smooth-walled high-profile gel implant, and it seems to promote better mobility of the implant in the periprosthetic capsular space.
I have extensive experience with textured shaped implants of both the saline-filled and silicone gel-filled types. Patients with long torsos and who require upper pole fullness are well served by tall implants such as the
INAMED Style 468 saline implant or the INAMED Style 410 textured shaped cohesive silicone gel device. My best results with saline implants with a wide variety of patient body types have come from the use of a shorter vertical dimension implant, namely the INAMED Style 363LF and the Contour Profile shaped textured saline device developed and marketed by Mentor Corp.
INAMED Style 468 saline implant or the INAMED Style 410 textured shaped cohesive silicone gel device. My best results with saline implants with a wide variety of patient body types have come from the use of a shorter vertical dimension implant, namely the INAMED Style 363LF and the Contour Profile shaped textured saline device developed and marketed by Mentor Corp.
With experience, good results can be obtained using almost any implant. At present my most consistent results are obtained with the use of a smooth-walled high-profile silicone gel-filled implant following preliminary tissue expansion with the short height tissue expander. I inform each patient before surgery that a breast reconstructed with an implant will not fill the anterior (front part) of her bra cup like her normal breast does. Furthermore, I tell patients preparing to undergo implant breast reconstruction that breasts that are reconstructed with implants will not look like, move like, or feel like their normal breasts.
DIFFICULT PATIENTS FOR IMPLANT-BASED BREAST RECONSTRUCTION
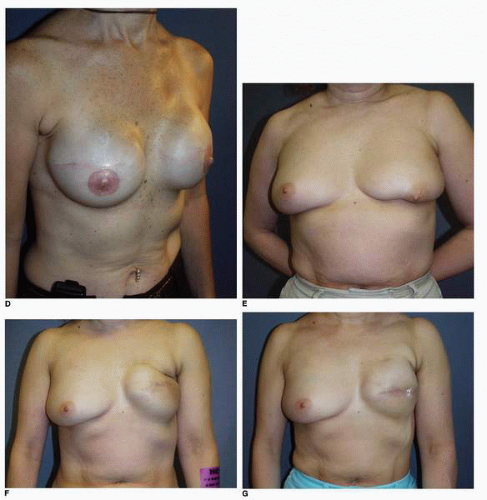
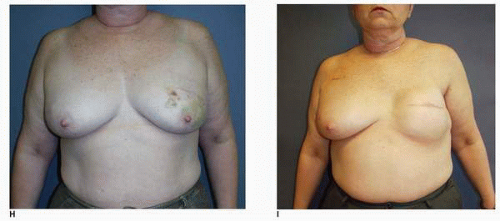
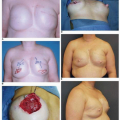
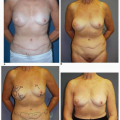
Synthetic implant-based reconstruction is often the default option for a patient who has insufficient donor tissue for an autogenous reconstruction. These patients are commonly of asthenic habitus and their chest wall tissues are very often thin (Fig. 6-11A). This situation often sets the stage for a suboptimal outcome following the tissue expansion process because the expansion itself further thins or attenuates the covering tissue (Fig. 6-11B). This scenario permits any implant irregularities to show through the skin (Fig. 6-11C,D). This type of patient must be informed before surgery of the limitations imposed by suboptimal qualitative and quantitative covering tissue at the site of implant breast reconstruction. Patients who have undergone previous lumpectomy and radiation or radiation of the skin following a mastectomy are also suboptimal implant reconstruction candidates (Fig. 6-11E). The radiated tissue does not expand easily (Fig. 6-11F), and in my experience there is a high rate of capsular contracture with the placement of any type of implant that often results in an unsatisfactory breast reconstruction (Fig. 6-11G). Finally, patients with a wide chest dimension, husky build, and heavy tissue in the chest region are also suboptimal candidates for implant reconstruction because they seem to swallow up the implants (Fig. 6-11H,I) and the tissue drape over the implants often fails to adequately replicate the opposite breast silhouette.
In summary, an essential aspect of achieving good aesthetic outcomes from implant-based breast reconstruction is patient selection. All too often implants are the default option. In such cases there are definite limitations imposed by the tissue conditions, and this reality must be communicated to the patient, preferably before surgery.
FIRST-STAGE BREAST RECONSTRUCTION WITH TISSUE EXPANSION USING SHORT-HEIGHT TISSUE EXPANDERS
For a long time I have believed that perhaps the main problem with tissue expansion for breast reconstruction is that too much stretching (and harmful tissue thinning) occurs where you do not need it or want it, namely in the tissues of the upper pole of the new breast. For that reason most recently I have used tissue expanders that predominantly [Contour Profile (Mentor Corp.); see Fig. 6-8A] or exclusively [LV low-height crescent expander (McGhan Medical Corp., Santa Barbara, Calif); see Fig. 6-8B] expand the lower pole tissues. These devices provide expansion where it is needed, i.e., predominantly in the lower pole. The tissue expander produces the appearance of a shelf or a ledge in the upper pole of the breast that is being created (Fig. 6-12). There is an additional benefit of not stretching the upper pole: a better breast shape at the second stage when the expander is removed and the implant is inserted, regardless of which implant is used. This is because the pectoralis major muscle (PMM) has not been stretched, and following the additional subpectoral dissection at the second stage the muscle compresses the implant, producing a straighter contour or tapered shape in the lateral view.
When I use a short-height tissue expander, I employ underexpansion. Specifically, I fill the expander to within 80% to 85% of the desired implant volume. This is because approximately the upper 30% of the new breast form is not generated as part of the expansion process, and the implant selected will generally have a volume that is 20% to 30% greater than that contained in the expander
at the completion of the filling process. I then give the tissues time to accommodate to their degree of stretch or expansion by allowing 8 to 12 weeks to elapse between the last expansion and the date of the implant exchange.
at the completion of the filling process. I then give the tissues time to accommodate to their degree of stretch or expansion by allowing 8 to 12 weeks to elapse between the last expansion and the date of the implant exchange.
At the time of tissue expander removal, a superior capsulotomy (Fig. 6-13A) is always needed if a short-height tissue expander has been used. This capsulotomy allows the surgeon to open and precisely develop the submuscular plane in the upper breast area, which has not undergone expansion (Fig. 6-13B). This allows the surgeon to create the desired fullness and shape of the upper pole by employing modifications of the surgical dissection in conjunction with the implant diameter, volume, and projection. If corrections of the IM fold or medial or lateral contours (Fig. 6-14) are needed after the expansion, these are done while performing the superior capsule release or capsulotomy.
In a very real sense this type of tissue expander permits the surgeon to control the upper pole shape of the newly reconstructed breast in a way not possible with other tissue expander designs. This is why it has been my
preferred method of tissue expansion for the vast majority of patients undergoing tissue expander breast reconstructions over the past 3 years. In my opinion it has increased the artistry and made this type of reconstruction more fun and fulfilling for both the patient and the surgeon.
preferred method of tissue expansion for the vast majority of patients undergoing tissue expander breast reconstructions over the past 3 years. In my opinion it has increased the artistry and made this type of reconstruction more fun and fulfilling for both the patient and the surgeon.
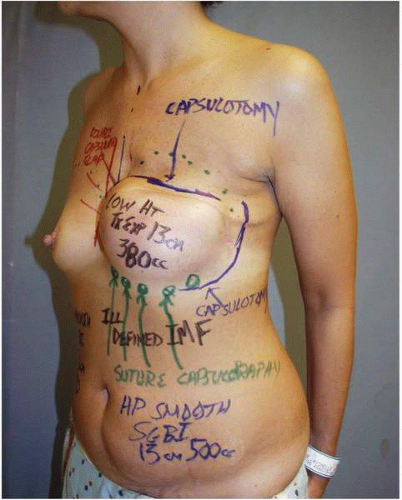 FIGURE 6-14. Additional modifications of capsule are outlined before surgery in two different patients. Such capsular modifications are very common and are completed at the time of expander removal, along with superior capsulotomy (Fig. 6-13B) and subpectoral dissection (Fig. 6-13C). |
I find it helpful to have sample tissue expanders and implants in the office for patients to examine. I also have schematic diagrams available so that patients can gain an insight into how the implant is to be positioned while they examine the integrated filler value and understand how it is filled. In addition, I find that it is helpful to show patients several samples of the type of implant that is most likely to be placed following the completion of the expansion process.
I believe that the technique used to place the tissue expander is very important. The device should be placed such that the lower pole accurately simulates the IM fold level of the opposite breast, and it should be placed in such a way that maximum skin recruitment and stretching (as opposed to muscle stretching) in the lower pole routinely occurs. Selecting an expander with the appropriate base dimension establishes the correct horizontal dimension of the periprosthetic capsular space and hence the correct horizontal silhouette of the new breast form. In this way the expansion process can shape and mold the surrounding soft tissue. Accurate device placement allows the tissue expansion process to generate a custom-made periprosthetic capsular space. This allows the preoperatively selected implant to be positioned in such a way to achieve the optimal breast shape.
The accuracy of the initial expander placement (at the first stage of the reconstruction) greatly facilitates a satisfactory outcome for most tissue expander/implant breast reconstructions. For that reason I will now describe my approach to the placement of a tissue expander in the setting of immediate breast reconstruction and also in the setting of a delayed breast reconstruction.
TECHNIQUE OF TISSUE EXPANDER PLACEMENT—IMMEDIATE RECONSTRUCTION
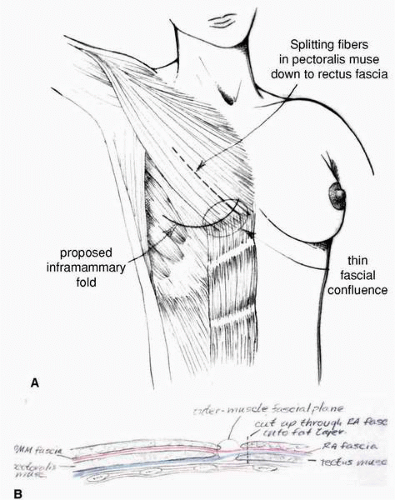
One of the most important factors in obtaining consistently good results with tissue expander breast reconstruction is maximizing the soft tissue coverage for the device. For me, this means the upper part of the expander (below the incision) is covered by the PMM and the lateral portion of the expander is covered by the serratus anterior muscle slips. The lower part of the device sits below the deep layer of the adipose tissue beneath an incision in the rectus abdominis muscle fascia medially and in the lateral two thirds of the IM fold just deep to the divided external oblique muscle (Fig. 6-15). This positioning is achieved in most cases of immediate breast reconstruction in the following way.
The marking for tissue expander insertion is straightforward. A skin-sparing mastectomy is preferred and performed in virtually every case. At our center the marks for the skin resection are designed by the plastic surgeon, but they are confirmed as oncologically sound by the surgeon performing the mastectomy. The most important landmark is the IM fold (Fig. 6-16). The lower limit of the dissection is either to the existing fold or to some level beneath it if the existing fold is too high relative to the opposite breast. The medial extent of the dissection is to the parasternal perforating vessels arising from the internal mammary artery. Laterally the dissection conducted in a plane below the serratus anterior muscle is usually performed to a site just posterior to the anterior axillary line. It can be extended as far posteriorly as the midaxillary line, however, if this is needed to accommodate the chosen expander. The plane above the ribs and beneath the serratus muscle over the lateral chest is quite distinct and easy to develop under direct vision. The necessary pocket dissection is dependent on the dimensions of the opposite breast when measured from the parasternal area
to the anterior axillary line, and it is developed to accommodate the selected tissue expander. The dissected space should be slightly larger than the height and width dimensions of the selected tissue expander.
to the anterior axillary line, and it is developed to accommodate the selected tissue expander. The dissected space should be slightly larger than the height and width dimensions of the selected tissue expander.
This dissection is done with the electrocautery device using the coagulation mode under illumination with a headlight or lighted retractor. The dissection proceeds inferiorly below the rectus abdominis muscle fascia. In immediate reconstruction it is imperative for the plastic surgeon to convey to the general surgeon performing the mastectomy the importance of not violating the pectoralis fascia in the lower aspect of the mastectomy dissection near its confluence with rectus fascia. In addition, the tissue between the PMM fascia and the rectus fascia, which I call the fascia intermedialis (see Fig. 6-15), should not be violated. If it is injured, it is very difficult to raise the rectus fascia confluent with the PMM fascia. If the PMM muscle is inadvertently injured or detached at its inferior origin, it most often can be repaired and placed back to its point of origin with 3-0 PDS suture (Ethicon, Inc., Somerville, NJ). If it cannot be sutured to its origin, it is often possible to suture it to the undersurface of the skin flap with 3-0 PDS suture, thereby providing muscle coverage for the tissue expander beneath the skin closure. Other surgeons have used 3-0 Prolene suture (Ethicon, Inc., Somerville, NJ) tied over a bolster to achieve the same goal of attaching the divided origin of the PMM to the inferior skin flap.8
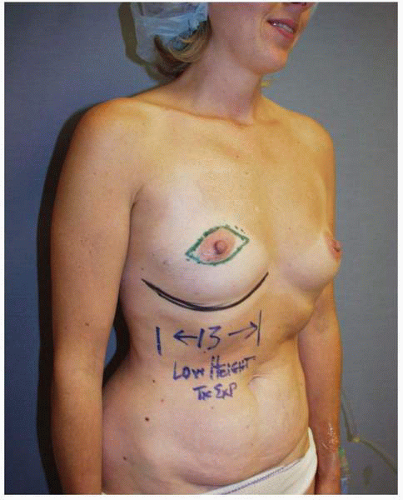 FIGURE 6-16. Marking patient for tissue expander insertion. The most important mark is the level of the IM fold. This patient will undergo a skin-sparing mastectomy. |
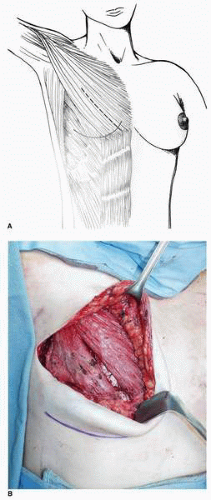
The subpectoral plane is accessed and developed by splitting the PMM in line with its fibers 3 cm medial to its lateral edge (Fig. 6-17A). The loose areolar layer beneath the PMM is readily visualized and the dissection proceeds medially, laterally, and superiorly. With the short-height expander, it is generally not necessary to dissect the subpectoral space beyond the third intercostal space.
During the initial dissection I find it easiest to identify the plane beneath the rectus fascia by extending the muscle-splitting incision in the PMM inferiorly over and through the rectus fascia (Fig. 6-17B) by simply
continuing it inferior-medially over and through the rectus fascia. When developing the plane deep to the PMM inferiorly, the medial PMM is elevated with the medial rectus muscle fascia. The lateral PMM is elevated in continuity with the lateral rectus fascia and accompanying external oblique muscle. The subfascial plane above the rectus muscle is developed in conjunction with the subexternal oblique plane as the dissection is carried laterally. Near the IM fold the plastic surgeon must gain access to the plane of the deep adipose layer above the rectus fascia by incising the fascia on its deep surface. At the desired level for the new IM fold (marked on the skin preoperatively) the dissection is brought more superficial, i.e., toward the skin. An incision is made with the electrocautery directed superficially through the deep surface of both the rectus fascia and external oblique muscles until the yellow adipose tissue at the deep surface of the deep layer of adipose tissue is seen (Fig. 6-18). This enables the tissue expansion process to generate the new IM fold in the most ideal position. Under direct vision the rectus fascia and external oblique muscle is incised on its deep surface in the shape of an arc that outlines the desired shape of the IM fold. Along this entire incision the surgeon will note a yellow layer of adipose tissue, and he or she should stop the dissection when this level is reached.
continuing it inferior-medially over and through the rectus fascia. When developing the plane deep to the PMM inferiorly, the medial PMM is elevated with the medial rectus muscle fascia. The lateral PMM is elevated in continuity with the lateral rectus fascia and accompanying external oblique muscle. The subfascial plane above the rectus muscle is developed in conjunction with the subexternal oblique plane as the dissection is carried laterally. Near the IM fold the plastic surgeon must gain access to the plane of the deep adipose layer above the rectus fascia by incising the fascia on its deep surface. At the desired level for the new IM fold (marked on the skin preoperatively) the dissection is brought more superficial, i.e., toward the skin. An incision is made with the electrocautery directed superficially through the deep surface of both the rectus fascia and external oblique muscles until the yellow adipose tissue at the deep surface of the deep layer of adipose tissue is seen (Fig. 6-18). This enables the tissue expansion process to generate the new IM fold in the most ideal position. Under direct vision the rectus fascia and external oblique muscle is incised on its deep surface in the shape of an arc that outlines the desired shape of the IM fold. Along this entire incision the surgeon will note a yellow layer of adipose tissue, and he or she should stop the dissection when this level is reached.
When this is completed, in addition to a line of yellow adipose tissue, the surgeon will note markedly increased distensibility of the entire inferior muscle-fascia envelope in the area of the lower pole under the inferior skin flap as the deep subcutaneous space is entered. It is in this plane that the lower pole of the tissue expander is positioned. If done properly there is complete coverage of the expander by the muscle layer above and the lower skin flap, including the rectus fascia and the external oblique muscle below.
The only patients in whom I do not make a concerted effort to release the rectus fascia and the external oblique muscle in this way are patients with exceedingly thin tissues, i.e., those patients with extremely little or virtually no subcutaneous adipose tissue. In such patients the external oblique muscle and rectus fascia are usually also thin, but they may provide a little bit of extra covering tissue or tissue padding, which may help camouflage the inferior edge of the implant. For that reason I may not extend the dissection superficially at the IM fold level in these patients.
A barrier drape of OpSite (Smith & Nephew, Largo, Fla; Chapter 3) is placed on the skin of the breast area and an opening is cut in it to permit the passage of the selected expander into the wound. In this way the tissue expander does not make contact with the skin. The expander is placed into position after the removal of the factoryinstilled air and without any fluid inside of it. As previously outlined, accurate positioning of the expander is
essential to facilitate symmetry. When it is in this flat-as-apancake (see Fig. 6-8) state, I believe that the most accurate positioning of the device is achieved. Next, sterile saline is introduced through a closed filling system.
essential to facilitate symmetry. When it is in this flat-as-apancake (see Fig. 6-8) state, I believe that the most accurate positioning of the device is achieved. Next, sterile saline is introduced through a closed filling system.
The head of the operating table is then brought as close to 90 degrees as possible so that the symmetry of the IM folds can be checked with the patient in the sitting position. The levels of the IM folds must be as symmetric as possible to facilitate overall breast symmetry. If such symmetry is not achieved, device repositioning or additional dissection or a combination of these maneuvers is done to achieve symmetric IM fold levels. As much fluid as possible is then placed into the expander while still permitting a tension-free closure of the skin wound. Closure of the PMM fibers ensures total deep tissue coverage of the device beneath the level of the skin incision.
This method is used for tissue expander placement in both immediate and delayed breast reconstruction. To reiterate an important point, the release of external oblique and rectus fascia places the lower pole of the tissue expander in the deep subcutaneous adipose space and this allows the expander to stretch and recruit skin rather than muscle. In my opinion this makes the process of tissue expansion for breast reconstruction quicker and less painful. In addition, placing the expander in the deep layer of adipose tissue allows the creation of a well-defined, natural-appearing lower breast pole.
Finally and most importantly, the use of short-height expanders has led to focusing the expansion process where it is needed—in the lower pole. In my experience the combination of using the described technique of tissue expander placement and the new short-height devices has resulted in implant-based breast reconstructions with a consistently better shape, marked by excellent lower pole aesthetics and a tapered upper pole (Fig. 6-19).
SURGICAL TECHNIQUE—DELAYED BREAST RECONSTRUCTION WITH TISSUE EXPANDER PLACEMENT
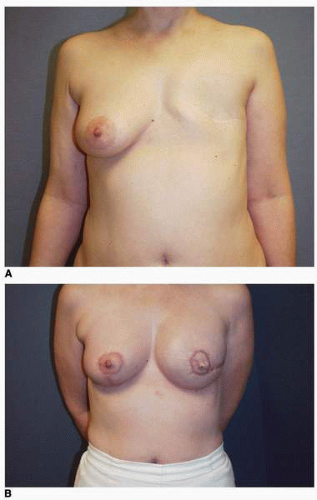
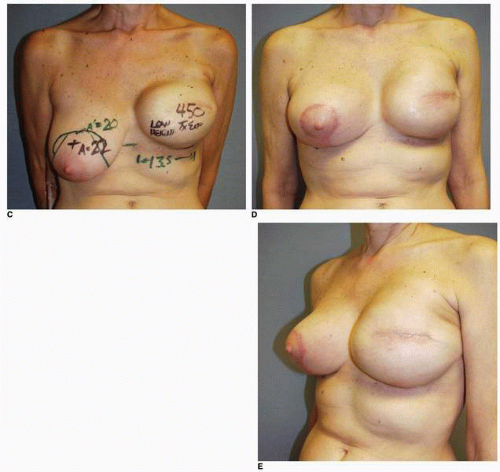
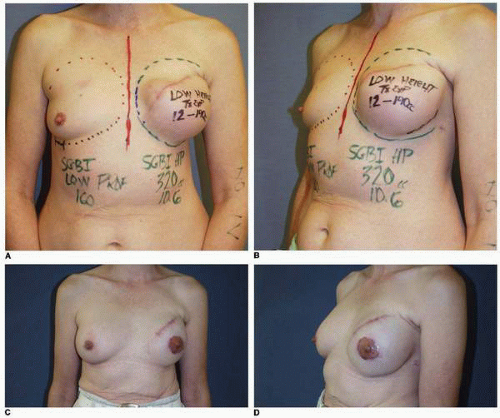
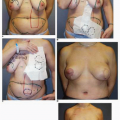
Preoperative patient marking is performed in every case exactly as described earlier. As in immediate reconstruction, the opposite breast serves as a template for the surgically absent breast in terms of IM fold level and base width (see Fig. 6-7). Most often only the central 60% of the mastectomy scar is incised and removed to permit exposure of the underlying PMM. However, if the scar shows hypertrophy, evidence of step-off, or other irregularity, it is entirely excised. Skin flaps are raised inferiorly and superiorly for a distance of 3 cm to expose the PMM fibers. The dissection of the subpectoral, subserratus space is then performed as described earlier and inferiorly the deep subcutaneous space (immediately superficial to the rectus fascia) is accessed. The dissection in the setting of a previous mastectomy is easier and generally proceeds more quickly than the same dissection done at the time of a mastectomy. This is because the scar tissue and healing following the mastectomy makes injury to or avulsion of the PMM exceedingly rare. In the case illustrated earlier (see Fig. 6-7) the plan was for a silicone gel implant reconstruction of the left postmastectomy defect with a partial subpectoral silicone gel breast augmentation of the contralateral right breast. A 12-cm base width tissue expander was used because it would accommodate a 12-cm 375-cc silicone gel implant on the left. (In addition, the plan called for a 160-cc silicone gel implant on the right [Fig. 6-20A,B].) Those implants were placed with the result displaying excellent shape and symmetry of the breasts (Fig. 6-20C,D).
ACUTE COMPLICATIONS FOLLOWING TISSUE EXPANDER AND IMPLANT PLACEMENT
The postoperative complications occurring in the acute phase following tissue expander placement and implant placement are bleeding, hematoma, seroma, infection, skin loss, implant exposure, and loss of the device. In general each of these should be managed aggressively to prevent loss of the expander.
Hematoma
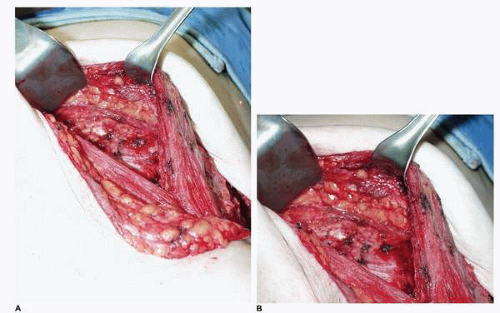
A collection of blood may occur in the submuscular or subcutaneous space. The surgeon routinely obtains meticulous hemostasis at the time of expander insertion. Despite this, hematomas can occur. This condition is either the result of a clot that comes off a blood vessel previously controlled at surgery or from a diffuse oozing that may be seen after the use of aspirin products. The former situation is more common and may occur following a cough or Valsalva maneuver. The most likely source is a small arterial branch of the internal mammary, lateral thoracic, or intercostal system. The patient usually presents with swelling, ecchymosis, and tenderness in the affected breast. I believe that returning the patient to the operating room to evacuate the hematoma is the best course. Most often a distinct bleeding point is not found. Nevertheless, the blood should be evacuated and a drain placed. This is the best chance of achieving a good reconstruction with the lowest probability of capsular contracture.
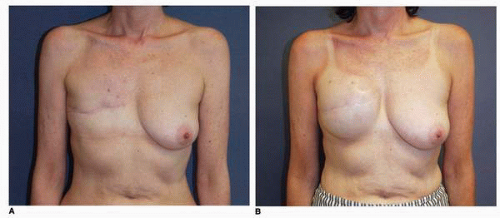
In addition, when a hematoma occurs in the subcutaneous space, it almost always imparts stiffness to the skin flap due to the fibrosis that results in response to the presence and the biologic resorption process of the blood collection in this space. Therefore in cases with significant hematomas (Fig. 6-21) I believe in the conservative treatment to reoperate to evacuate the blood and to place a drain (Fig. 6-21B). In this way the surgeon creates the best possible softness and draping potential of the skin flap over the implant placed at the second stage.
Such a case is illustrated by this patient who presented with swelling and ecchymosis of the left breast 1 week following an immediate tissue expansion reconstruction of the left breast (Fig. 6-21A). She underwent operative exploration and removal of blood from the subcutaneous space (Fig. 6-21B). The tissue expander in the submuscular position had been completely covered by the PMM and was preserved. She underwent successful bilateral tissue expansion, and subsequent implant placement produced a good outcome for her in terms of her bilateral breast reconstruction (Fig. 6-21C). I believe blood around an implant probably increases the risk of capsular contracture in the setting of both breast reconstruction and breast augmentation. As also noted, the additional fibrosis in the skin flap that often occurs in this setting limits the optimal drape of the skin flap over the implant, thereby limiting the aesthetic outcome. Therefore, it is my custom to evacuate any significant collection that occurs.
Seroma
Seromas are not uncommon, especially following immediate breast reconstruction with tissue expander placement. It is most likely due to the extensive dissection of the subcutaneous space during elevation of the mastectomy flaps or during axillary dissection. Although a drain is placed into this space in virtually all cases and some element of tamponade is provided by the saline fill of the expander, seromas still occur. They are usually managed by sterile needle aspiration of the subcutaneous space with the needle being placed over the area of the filler port of the expander but not being advanced to the point where it penetrates the port (Fig. 6-22A). The fluid can be compressed or moved to the area immediately over the filler port by an assistant’s hand (Fig. 6-22B). Following aspiration of the seroma fluid, saline is installed into the expander to obliterate the space into which it might reaccumulate. It may be necessary to perform this sequence several times before the problem is resolved.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree